User:Milton Beychok/Sandbox
In chemistry and physics, volatility is a term used to characterize the tendency of a substance to vaporize.[1] It is directly related to a substance' s vapor pressure. At a given temperature, a substance with a higher vapor pressure will vaporize more readily than a vapor with a lower vapor pressure.[2][3][4]
In common usage, the term applies primarily to liquids. However, it may also be used to characterize the process of sublimation by which certain solid substances such as ammonium chloride (NH4Cl) and dry ice, which is solid carbon dioxide (CO2), change directly from their solid form to a vapor without becoming a liquid.
Any substance with a significant vapor pressure at temperatures of about 20 to 25 °C (68 to 77 °F) is very often referred to as being volatile.
Vapor Pressure, temperature and boiling point
The vapor pressure of a substance is the pressure at which its gaseous (vapor) phase is in equilibrium with its liquid or solid phase. It is a measure of the tendency of molecules and atoms to escape from a liquid or solid. A liquid's boiling point at atmospheric pressure corresponds to the temperature at which its vapor pressure is equal to the surrounding atmospheric pressure and is very commonly referred to as the normal boiling point.
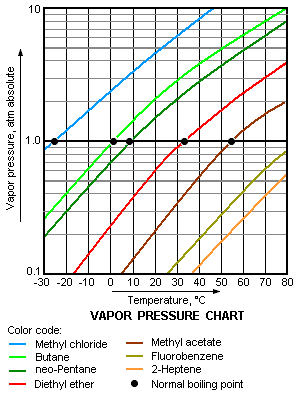
The higher is the vapor pressure of a liquid, the higher is the volatility and the lower is the normal boiling point of the liquid. The adjacent vapor pressure chart graphs the dependency of vapor pressure upon temperature for a variety of liquids[5] and also confirms that liquids with higher vapor pressures have lower normal boiling points.
For example, at any given temperature, methyl chloride (CH3Cl) has the highest vapor pressure of any of the liquids graphed in the chart. It also has the lowest normal boiling point (−26 °C), which is where its vapor pressure curve (the blue line) intersects the horizontal pressure line of one atmosphere (atm) of absolute vapor pressure.
Relative volatility
{{main|Relative volatility)
Relative volatility is a measure of the difference between the vapor pressure of the more volatile components of a liquid mixture and the vapor pressure of the less volatile components of the mixture. This measure is widely used in designing large industrial distillation processes.[4][5][6] In effect, it indicates the ease or difficulty of using distillation to separate the more volatile components from the less volatile components in a mixture. In other words, the higher is the relative volatility of a liquid mixture, the easier it is to separate the mixture components by distillation. By convention, relative volatility is typically denoted as .
References
- ↑ Note: To vaporize means to become a vapor.
- ↑ Gases and Vapor (University of Kentucky website)
- ↑ James G. Speight (2006). The Chemistry and Technology of Petroleum, 4th Edition. CRC Press. ISBN 0-8493-9067-2.
- ↑ 4.0 4.1 Kister, Henry Z. (1992). Distillation Design, 1st Edition. McGraw-Hill. ISBN 0-07-034909-6.
- ↑ 5.0 5.1 R.H. Perry and D.W. Green (Editors) (1997). Perry's Chemical Engineers' Handbook, 7th Edition. McGraw-Hill. ISBN 0-07-049842-5.
- ↑ Seader, J. D., and Henley, Ernest J. (1998). Separation Process Principles. New York: Wiley. ISBN 0-471-58626-9.
Bibliography:
- Lawrence K. Wang, Norman C. Pereira and Yung-Tse Hung (Editors) (2004). Air Pollution Control Engineering, 1st Edition. Humana Press. ISBN 1-58829-161-8.