User:Milton Beychok/Sandbox
Air stripping is the transferring of Volatile Organic Compounds (VOC) and/or other volatile components of a liquid into an air stream. It is a chemical engineering process used for the purification of groundwaters and wastewaters containing volatile compounds.[1][2]
Air stripping for the removal of contaminants from groundwaters and wastewaters is especially effective for contaminants that have a high vapor pressure and a low solubility inwater.[1][2]
The compounds removed by air stripping include benzene, toluene, ethylbenzene, and xylene (BTEX) and various solvents (such as trichloroethylene and tetrachloroethylene) found in some wastewaters. Ammonia can also be stripped from wastewaters.
Process description
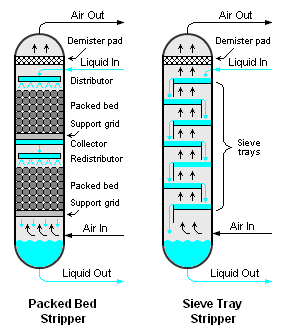
Air stripping is usually accomplished by using either a packed or trayed vertical vessel[3][4][5] as shown in Figure 1. Either type of air stripper involves the downward flow of a volatile-containing liquid (most often, water) and the upward flow of air. The liquid and air are intimately contacted by the packing or the trays in the stripper. The volatile components in the liquid are transferred into the air stream by the series of intimate contacts occurring at each tray as the air flows upward through the stripper.
A properly designed and operated air stripper can remove up to 99.9+ percent of volatile organic compounds (VOC) from groundwaters and wastewaters.
There are many different types of trays, including bubble-cap trays, valve-cap trays and sieve trays. Sieve trays are the most commonly used type in air strippers because they are very simple, less expensive than the other types and adequately efficient for use in air strippers.[6] There are also many types of packing available for packed bed strippers, either randomly packed or structurally packed material as shown in the adjacent photographs.
The stripped liquid leaving the bottom of the stripper is essentially free of volatiles. The air stream leaving the top of the stripper contains the volatiles removed from the liquid and may require some type of emissions mitigation to comply with applicable environmental regulatory limits such as the National Emissions Standards for Hazardous Air Pollutants (HAP) as set by the U.S. Environmental Protection Agency.
When mitigation of the air pollutant emissions from an air stripper is required, there are three technologies available:
- Adsorption in activated carbon filters
- Catalytic oxidation
- Incineration also known as thermal oxidation
The relative effectiveness of the above mitigation technologies depend to a great extent on the concentration level of the pollutants in the air stream from an air stripper. Very low pollutant concentrations mean that very large volumes of air must be handled in order to mitigate very small amounts of pollutants. In some cases, it would be prudent to perform air pollution dispersion modeling studies to determine whether or not routing the air stream through a vent stack of a reasonable height would result in compliance with the applicable environmental regulations.
Design and operational variables
(Just some notes. This section is still to be organized and written.)
The variables and design considerations for strippers are many. Among them are the entering conditions, the degree of recovery of the solute needed, the choice of the stripping agent and its flow, the operating conditions, the number of stages, the heat effects, and the type and size of the equipment.[3]
The degree of recovery is often determined by environmental regulations, such as for volatile organic compounds like chloroform.
As stripping agents are gases, operation at nearly the highest temperature and lowest pressure that will maintain the components and not vaporize the liquid feed stream is desired. This allows for the minimization of flow. As with all other variables, minimizing cost while achieving efficient separation is the ultimate goal.
The size of the equipment, and particularly the height and diameter, is important in determining the possibility of flow channeling that would reduce the contact area between the liquid and vapor streams. If flow channeling is suspected to be occurring, a redistribution plate is often necessary to, as the name indicates, redistribute the liquid flow evenly to reestablish a higher contact area.
During operation, monitoring the pressure drop across the column can help to determine the performance of the stripper. A changed pressure drop over a significant range of time can be an indication that the packing may need to be replaced or cleaned.
Typical applications
References
- ↑ 1.0 1.1 Kathleen Sellers (1998). Hazardous Waste Site Remediation, 1st Edition. CRC Press, pages 131 - 134.
- ↑ 2.0 2.1 Air Stripping of VOCs from Water From the website of Jaeger Products, Inc.
- ↑ 3.0 3.1 J.D. Seader and E.J. Henley (2006). Separation Process Principles, 2nd Edition. John Wiley & Sons. ISBN 0-471-46480-5.
- ↑ P. Chattpadhyay (2007). Absorption and Stripping, 1st Edition. Asian Books Pvt.Ltd., pages 2.98 - 2.116. ISBN 81-8412-033-8.
- ↑ E. Roberts Alley (2007). Water Quality Control Handbook, 2nd Edition. McGraw Hill, pages 9.48 - 9.56. ISBN 0-07-146760-2.
- ↑ Note: Sieve trays are simply metal plates perforated with many small holes through which the air flows upward to come into intimate contact with the liquid flowing across the plates. See Figure 1.
External links:
- Process Design Manual for Stripping of Organics Harish M. Shukla and R. Edwin Hicks (1984), U.S. EPA Report EPA-600/2-84-134
- Application of Sieve-Tray Air Strippers to the Treatment of Surfactant-Containing Wastewaters Tohren C. G. Kibbey, Kurt D. Pennell and Kim F. Hayes (2001), AIChE Journal, Vol. 47, No. 6.
- Engineering Design: Air Stripping U.S. Army Corps of Engineers Design Guide 1110-1-3, October 31, 2001
Bibliography:
- American Water Works Association (2003). Water Treatment, 3rd Edition. American Water Works Association. ISBN 1-58321-230-2.
- J. Russell Boulding (Editor) (1996). EPA Environmental Engineering Sourcebook, 1st Edition. CRC Press. ISBN 1-57504-002-6.