User:Milton Beychok/Sandbox
A jumble of notes for an article on air and steam strippers
Air stripping is the transferring of Volatile Organic Compounds (VOC) and/or other volatile components of a liquid into an air stream. It is a chemical engineering process used for the purification of groundwaters and wastewaters containing volatile compounds.
The compounds removed by air stripping include benzene, toluene, ethylbenzene, and xylene (BTEX) and various solvents (such as trichloroethylene and tetrachloroethylene) found in some wastewaters. Ammonia can also be stripped from wastewaters.
Equipment
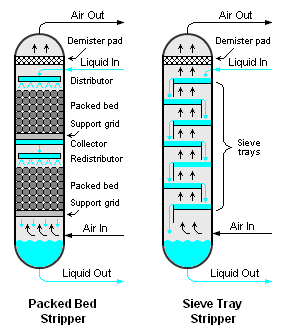
Air stripping is usually accomplished by using either a packed or trayed vertical vessel[1] as shown in Figure 1. Either type of air stripper involves the downward flow of a volatile-containing liquid (most often, water) and the upward flow of air. The liquid and air are intimately contacted by the packing or the trays in the stripper.
The stripped liquid leaving the bottom of the stripper is essentially free of volatiles. The air stream leaving the top of the stripper contains the volatiles removed from the liquid and may require some type of emissions mitigation to comply with applicable environmental regulatory limits such as the National Emissions Standards for Hazardous Air Pollutants (HAP) as set by the U.S. Environmental Protection Agency.
Carbon adsorption is often used and catalytic oxidation is another option.
Design and operational variables
The variables and design considerations for strippers are many. Among them are the entering conditions, the degree of recovery of the solute needed, the choice of the stripping agent and its flow, the operating conditions, the number of stages, the heat effects, and the type and size of the equipment.[1]
The degree of recovery is often determined by environmental regulations, such as for volatile organic compounds like chloroform.
Frequently, steam, air, inert gases, and hydrocarbon gases are used as stripping agents. This is based on solubility, stability, degree of corrosiveness, and availability. As stripping agents are gases, operation at nearly the highest temperature and lowest pressure that will maintain the components and not vaporize the liquid feed stream is desired. This allows for the minimization of flow. As with all other variables, minimizing cost while achieving efficient separation is the ultimate goal.[1]
The size of the equipment, and particularly the height and diameter, is important in determining the possibility of flow channeling that would reduce the contact area between the liquid and vapor streams. If flow channeling is suspected to be occurring, a redistribution plate is often necessary to, as the name indicates, redistribute the liquid flow evenly to reestablish a higher contact area.
As mentioned previously, strippers can be trayed or packed. Packed columns, and particularly when random packing is used, are usually favored for smaller columns with a diameter less than 2 feet and a packed height of not more than 20 feet. Packed columns can also be advantageous for corrosive fluids, high foaming fluids, when fluid velocity is high, and when particularly low pressure drop is desired. Trayed strippers are advantageous because of ease of design and scale up. Structured packing can be used similar to trays despite possibly being the same material as dumped (random) packing. Using structured packing is a common method to increase the capacity for separation or to replace damaged trays.[1]
Trayed strippers can have sieve, valve, or bubble cap trays while packed strippers can have either structured packing or random packing.[1] Trays and packing are used to increase the contact area over which mass transfer can occur as mass transfer theory dictates. Packing can have varying material, surface area, flow area, and associated pressure drop. Older generation packing include ceramic Raschig rings and Berl saddles. More common packing materials are metal and plastic Pall rings, metal Michael Bialecki rings, and ceramic Intalox saddles. Each packing material of this newer generation improves the surface area, the flow area, and/or the associated pressure drop across the packing. Also important, is the ability of the packing material to not stack on top of itself. If such stacking occurs, it drastically reduces the surface area of the material. Lattice design work has been increasing of late that will further improve these characteristics.[1]
During operation, monitoring the pressure drop across the column can help to determine the performance of the stripper. A changed pressure drop over a significant range of time can be an indication that the packing may need to be replaced or cleaned.
Typical applications
Steam is also frequently used as a stripping agent for water treatment. Volatile organic compounds are partially soluble in water and because of environmental considerations and regulations, must be removed from groundwater, surface water, and wastewater.[2] These compounds can be present because of industrial, agricultural, and commercial activity.
References
- Henry Z. Kister (1992). Distillation Design, 1st Edition. McGraw-Hill. ISBN 0-07-034909-6.
- Perry, R.H. and Green, D.W. (Editors) (1997). Perry's Chemical Engineers' Handbook, 7th Edition. McGraw-Hill. ISBN 0-07-049841-5.
External links:
- Air Stripping from a website page of the Federal Remediation Technologies Roundtable
- Air Stripping of VOCs from Water
- Air Stripping, Design Guide 1110-1-3 from website page of the U.S. Army Corps of Engineers