Thiophosphoramidite: Difference between revisions
imported>David E. Volk m (fixed bad structure picture) |
mNo edit summary |
||
| Line 2: | Line 2: | ||
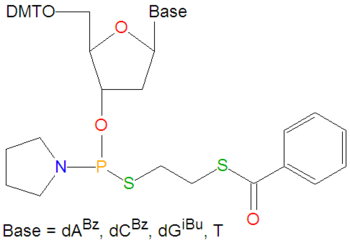
{{Image|Thiophosphoramidites.png|right|350px|Thiophosphoramidites used to make DNA containing dithioate linkages.}} | {{Image|Thiophosphoramidites.png|right|350px|Thiophosphoramidites used to make DNA containing dithioate linkages.}} | ||
'''Thiophosphoramidites''' are a class of reagents typically used in [[DNA]] [[DNA synthesizer|synthesizers]] for making DNA strands which have two sulfur atom substitutions in place of the non-bridging oxygen atoms of the phosphate linker between the O3' and O5' oxygen atoms between two nucleotides. In such DNA, one of the sulfur atoms originates from the thiophosphoramidite and the second is derived from a sulfurizing "oxidizing" agent, such as the [[Beaucage reagent]]. These compounds are used to make [[thioaptamer]]s, DNA or RNA molecules synthesized to bind to protein targets, either by design or random library selection. Like the normal [[phosphoramidites]] used in DNA synthesizers, the bases of these molecules must be protected during the nucleotide coupling steps during DNA synthesis, and the blocking groups are then removed when the completed DNA strand has been synthesized. Thiophosphoramidites, and some reactions leading up to them, produce a ''very'' strong and unpleasant odor. | '''Thiophosphoramidites''' are a class of reagents typically used in [[DNA]] [[DNA synthesizer|synthesizers]] for making DNA strands which have two sulfur atom substitutions in place of the non-bridging oxygen atoms of the phosphate linker between the O3' and O5' oxygen atoms between two nucleotides. In such DNA, one of the sulfur atoms originates from the thiophosphoramidite and the second is derived from a sulfurizing "oxidizing" agent, such as the [[Beaucage reagent]]. These compounds are used to make [[thioaptamer]]s, DNA or RNA molecules synthesized to bind to protein targets, either by design or random library selection. Like the normal [[phosphoramidites]] used in DNA synthesizers, the bases of these molecules must be protected during the nucleotide coupling steps during DNA synthesis, and the blocking groups are then removed when the completed DNA strand has been synthesized. Thiophosphoramidites, and some reactions leading up to them, produce a ''very'' strong and unpleasant odor. | ||
[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 06:01, 28 October 2024
Thiophosphoramidites are a class of reagents typically used in DNA synthesizers for making DNA strands which have two sulfur atom substitutions in place of the non-bridging oxygen atoms of the phosphate linker between the O3' and O5' oxygen atoms between two nucleotides. In such DNA, one of the sulfur atoms originates from the thiophosphoramidite and the second is derived from a sulfurizing "oxidizing" agent, such as the Beaucage reagent. These compounds are used to make thioaptamers, DNA or RNA molecules synthesized to bind to protein targets, either by design or random library selection. Like the normal phosphoramidites used in DNA synthesizers, the bases of these molecules must be protected during the nucleotide coupling steps during DNA synthesis, and the blocking groups are then removed when the completed DNA strand has been synthesized. Thiophosphoramidites, and some reactions leading up to them, produce a very strong and unpleasant odor.