Tetrazole: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk mNo edit summary |
mNo edit summary |
||
| Line 3: | Line 3: | ||
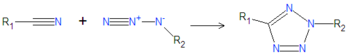
{{Image|Tetrazole Click chemistry.png|right|350px|Tetrazole formed by cyclization of a cyanide and an azide.}} | {{Image|Tetrazole Click chemistry.png|right|350px|Tetrazole formed by cyclization of a cyanide and an azide.}} | ||
[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 06:00, 26 October 2024
Tetrazoles are cyclic chemical compounds having a base structure (C1N4H2) in which four nitrogen atoms and one carbon atom form a 5-atom heterocycle. They can be synthesized by click chemistry, reacting a cyanide with an azide, as depicted below. The base tetrazole compound (R1 = R2 = H) is commonly used as a base in chemical reactions. Some tetrazoles are angiotensin receptor antagonists used to treat high blood pressure (hypertension).