Aluminium trichloride: Difference between revisions
Jump to navigation
Jump to search

imported>Meg Taylor (update) |
mNo edit summary |
||
| Line 20: | Line 20: | ||
== References == | == References == | ||
{{reflist}} | {{reflist}}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 06:01, 9 July 2024
|
| |||||||
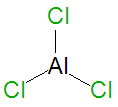
| Aluminium trichloride | |||||||
| |||||||
| Uses: | catalyst, astringent | ||||||
| Properties: | pyrophoric Lewis acid | ||||||
| Hazards: | spontaneously ignites in air | ||||||
| |||||||
Aluminium trichloride, AlCl3, is an important industrial chemical catalyst, especially for polymerization reactions. It is a pyrophoric Lewis acid chemical compound which instantly ignites when it is exposed to air. It is a highly reactive compound that can also be used as a chemical promoter in chelation-controlled reactions. It is also an astringent, used medically for athlete's foot hyperhidrosis therapy.[1]