Systems biology: Difference between revisions
imported>Anthony.Sebastian |
imported>Anthony.Sebastian m (Ref #1) |
||
| Line 1: | Line 1: | ||
As an academic discipline, '''systems biology''' aims to explain, predict and control the properties, functions and behaviors<ref>''Property'': quality or trait peculiar to a thing (e.g., mass, volume, ability to reproduce, structure, lifespan, etc.; ''function'': action specially fitted for a thing (e.g., locomotion, phagocytosis, phototropism, etc.); ''behavior'': the activity detected by the observer (e.g., deception, flight, chemotaxis, etc.). The distinctions among those often blur, ‘''property''’ serving generically in many instances.</ref> of biological, or living, [[system]]s—compartmentalized assemblages of interrelated, dynamically interacting, coordinated and hierarchically organized components.<ref name=kitano2002a>Kitano H (2002) [http://www.sciencemag.org/cgi/content/full/295/5560/1662/ Systems biology: a brief overview] ''Science'' 295:1662-1664 PMID 11872829</ref> | As an academic discipline, '''systems biology''' aims to explain, predict and control the properties, functions and behaviors<ref>''Property'': quality or trait peculiar to a thing (e.g., mass, volume, ability to reproduce, structure, lifespan, etc.; ''function'': action specially fitted for a thing (e.g., locomotion, phagocytosis, phototropism, functioning as a molecular motor, energy transduction, etc.); ''behavior'': the activity detected by the observer (e.g., deception, flight, chemotaxis, etc.). The distinctions among those often blur, ‘''property''’ serving generically in many instances.</ref> of biological, or living, [[system]]s—compartmentalized assemblages of interrelated, dynamically interacting, coordinated and hierarchically organized components.<ref name=kitano2002a>Kitano H (2002) [http://www.sciencemag.org/cgi/content/full/295/5560/1662/ Systems biology: a brief overview] ''Science'' 295:1662-1664 PMID 11872829</ref> | ||
Developing models (aka representations) of various kinds, including quantitative and computer-based models, systems biologists try to achieve those aims in part through analyses of experimentally derived data about a system’s interacting components. They often work with non-biological scientists to develop realistic models of biological systems that can allow predictions of their properties, functions and behaviors in response to given stimuli or given sets of conditions. They use sophisticated mathematical, statistical and computational tools in diverse modeling approaches, in network analyses, in computer simulations, in design and building synthetic networks; and, iteratively, incorporating new data derived from systems-analysis-inspired further experimentation. Modeling permits a kind of formal integrative analysis of a living system or subsystem. | Developing models (aka representations) of various kinds, including quantitative and computer-based models, systems biologists try to achieve those aims in part through analyses of experimentally derived data about a system’s interacting components. They often work with non-biological scientists to develop realistic models of biological systems that can allow predictions of their properties, functions and behaviors in response to given stimuli or given sets of conditions. They use sophisticated mathematical, statistical and computational tools in diverse modeling approaches, in network analyses, in computer simulations, in design and building synthetic networks; and, iteratively, incorporating new data derived from systems-analysis-inspired further experimentation. Modeling permits a kind of formal integrative analysis of a living system or subsystem. | ||
Revision as of 14:50, 24 February 2007
As an academic discipline, systems biology aims to explain, predict and control the properties, functions and behaviors[1] of biological, or living, systems—compartmentalized assemblages of interrelated, dynamically interacting, coordinated and hierarchically organized components.[2]
Developing models (aka representations) of various kinds, including quantitative and computer-based models, systems biologists try to achieve those aims in part through analyses of experimentally derived data about a system’s interacting components. They often work with non-biological scientists to develop realistic models of biological systems that can allow predictions of their properties, functions and behaviors in response to given stimuli or given sets of conditions. They use sophisticated mathematical, statistical and computational tools in diverse modeling approaches, in network analyses, in computer simulations, in design and building synthetic networks; and, iteratively, incorporating new data derived from systems-analysis-inspired further experimentation. Modeling permits a kind of formal integrative analysis of a living system or subsystem.
No definition or succinct description can capture the breadth and depth of the interdisciplinary enterprise of systems biology. Indeed, historian and philosopher of science Evelyn Fox Keller argues that “so far, ‘systems biology’ is a concept waiting for definition".[3] This article elaborates on the above description of the discipline.
Methodologies In General Terms
Systems biologists try to accomplish their aims in part:
- by studying the interrelations (structural) and interactions (dynamical, coordinated, hierarchical) among various components of a biological system; for example, (a) molecular components: gene and protein interactions involved in a cell’s metabolic pathways; (b) organism components: predator and prey behaviors in an ecosystem;
- by analyzing attempting to organize the system’s components in the form of models/simulations, which encode such concepts as modules, circuits and networks, feedback and feedforward loops, homeostasis, hierarchies, robustness, complexity, adaptation, and emergence;
- by further experiments to define interrelations and interactions of the real system that the model/simulation appeared to need for better fidelity;
- by refining the model/simulation on the basis of the data acquired by the further experiment;
- by design, construction and testing of synthetic networks.
Systems biologists expect progress in the field to yield explanations of biological systems suitable for applications in ecology, ethology, medicine, agriculture, business, and technology--and to a considerable extent it has already done so. Some systems biologists consider the discipline critical to further progress in biology.[4]
The study of biological systems has expanded greatly as the result of major advances in molecular biology in the late 20th and early 21st century. Those include sequencing of genomes, developments in genetic engineering, and developments of technologies for generating massive amounts of data on the structure and interactions of cellular components---all fueled by increasing interest by non-biological scientists (mathematicians, computer scientists, physicists, chemists, and engineers, among others) in applying the principles and methods of their sciences to the explanation of the adaptive complexity of living systems (see Figure 1).
On the Nature of Biological "Systems"
A 'system' in biology is any interconnected, interacting, coordinated and hierarchical assembly of biological components or elements—a functioning, organized assembly. For example, the vertebrate body is an assembly of diverse, interacting organs, among other components. See other examples below. Each component in a biological system interacts in some way(s) with one or more components in the system--a dynamical assembly of components. For example, in the system constituting a cell, proteins are the products of genes, but they also interact with genes, affecting their expression, as well as with other proteins. Systems can exhibit behaviors that are characteristic of the system-as-a-whole (see below), but which are not shared by any of its components (so-called emergent behaviors). A tree fruits, for example, because its dynamically interacting components enable it to, but no single component of a tree can. (More on emergent behavior below.)
Subsystems consist of smaller (less complex) systems embedded in a larger (more complex) system, and constitute at least part of the components or elements of the larger system. Whether a systems biologist treats a given assembly of components as a subsystem or as a system depends on the 'level' at which she focuses her attention. If she focuses her research at the level of a whole vertebrate organism, for example, she treats its organs as subsystems. If she focuses her research at the level of the heart, she treats the heart's interacting assemblage of components as a system, recognizing that the heart system remains a component or element of a larger system (e.g., the circulatory system).
Even the larger systems, e.g., the vertebrate body system, function as components or elements of even larger systems, a species of vertebrates, say, where individual members of the species interact with each other, as components, to generate a set of behaviors or properties characteristic of the species but not of the individual members of the species. The flocking behavior of birds illustrates a species behavior--technically the behavior of a deme. One bird cannot flock.
When trying to understand biological systems, systems biologists need not treat the components or elements of a system (or subsystem) exclusively as discrete or concrete objects or entities (e.g., molecules, organelles, cells, etc.), but may also treat them as abstracted concepts of organizational collections or activity patterns of those objects or entities, admitting of study by mathematical, computational and statistical tools. Those include such concepts as circuits, networks and modules, more about which will follow below. Such concepts have a way of appearing less abstract or hypothetical as biologists more fully define them in terms of structure and coordinated dynamical interactions; predict systems behavior from them using quantitative models; and relate them functionally in the larger systems embedding them.
Biological system behaviors typically perform one or more evolution-informed functions (e.g., growth), so unravelling the evolutionary history of a biological system contributes importantly in fully explaining it.
Examples of biological systems (subsystems) include:
- the biosphere
- ecosystems (e.g., a forest)
- species (e.g., Homo sapiens)
- demes (e.g., a local population of a species)
- organisms (e.g., humans; bacteria)
- organs (e.g., brain; the vascular endothelium)
- cells (e.g., epithelial cell)
- metabolic pathways (e.g., glycolysis)
- genomes (e.g., the entire complement of DNA in an organism, as the ’mouse genome')
- gene complexes (e.g., co-expressing genes)
- genes (e.g., protein blueprints)
History of Systems Biology
Knowledge of the historical path(s) leading to a modern scientific program offers a perspective that contributes heuristically to an understanding of the nature of the program, in particular, its goals and its methodologies. So for systems biology. In part, the facilitation to understanding results from the different focuses and approaches different historians have in writing about the same topic.
The Evolution of Molecular Biology into Systems Biology
Westerhoff and Palsson[4] introduce the school of thought “…that systems biology of the living cell has its origin in the expansion of molecular biology to genome-wide analyses.” They point out, however, that molecular biology has an earlier history of systems thinking, in particular in the elucidation of numerous molecular regulatory circuits and of their contribution to the logic of the cell. They see technologic advances that have permitted rapid collection of large data sets (so-called high-throughput technologies mapping the genome) as upping the scale of that research “…enabling us [molecular biologists] to view the genome as the ‘system’ to study.”
They describe two separate pathways of enquiry that they see as having merged into modern systems biology of the cell.
They see one root as the advances in molecular biology that ultimately “…led to efforts toward genome-scale model building to analyze the systems properties of cellular function”: recognition of DNA as the genetic material, identifying the structure of DNA, recombinant technology, automated determination of DNA base sequences, and high-throughput technologies yielding the sequences of entire genomes.
They discuss as a second, parallel root the development of non-equilibrium thermodynamics, a predictive mathematical theory for describing the behavior of systems that transfer energy from one place to another or convert energy from one form to another in processes the move towards an irreversible state of stability characterized by randomness or disorder—for biological systems, death. Since living systems produce order and maintain a state of non-randomness they must carry out processes that keep them in a condition far from the equilibrium of randomness, which they achieve by transforming energy (and matter and information) taken in from the environment. The non-closed biological system produces order at the expense of the environment it opens to, which environment becomes more disordered.
Westerhoff and Palsson describe advances in the development of non-equilibrium thermodynamics as presaging molecular and cellular systems biology through ‘quantitative’ integration of system components and through the discovery of principles connecting molecular mechanisms and system behaviors. Westerhoff and Alberghina[5] ask of systems biology: “Did we know it all along?”
Historical Milestones in the Development of Systems Biology
Anthony Trewavas, a scientist conducting research in the molecular and systems biology of plant cells, emphasizes the following aspects of the history of systems biology:[6]
- How Rene Descartes’ formulation of reductionism—the concept that the properties of complex objects can be explained by reducing them into their parts and studying the properties of the parts —led biologists to assume that one could explain a complex biological system's behavior from the behavior of its subsystems;
- How reductionism played hand-in-hand with the mechanistic view of reality, leading biologists to view systems as predetermined machines, like clocks;
- How reaction against the reductionist-mechanistic view led to a holistic view of biological systems consistent with Aristotle’s dictum, commonly expressed as "the whole is greater than the sum of its parts";
- How experiments revealed that the averaged behavior of a population did not apply to individual members of the population, however homogeneous the population appeared, complementing experiments revealing non-machine-like toleration of large variability among kindred organisms, organs and cells with respect to behavior and function—each manifesting a so-called norm of reaction that overlapped among individuals;
- How a system’s organization of subsystems itself exerts a level of control and constraint over the range of behaviors available to the subsystems in isolation;
- How a system’s subsystems are organised in hierarchies, where the properties of a subsystem emerge from the dynamic interactions of subsystems at lower levels of the hierarchy;
- How subsystems at lower levels of hierarchy exhibit more variability and how their behavior exhibits more order within the system than without—the higher level emergent properties orchestrate and constrain the behavior of the subsystems generating that emergence;
- How Karl Ludwig von Bertalanffy [b. 1901, d. 1972] recognized that all systems share “…the common property of being composed of interlinked components, in which case they might share similarities in detailed structure and control design”;
- How Michael Polyani (1891-1976) recognized that adjacent levels of a system’s subsystems constrain each other and that upper level behaviors require the lower level behaviors;
- How psychologist and polymath Donald T. Campbell (1916-1996) coined the term “downward causation” to describe higher system level constraints on lower levels, as in constraints on gene expression by higher level subsystems—a prelude to understanding ‘emergent’ systems as having properties or behaviors that “make a difference”, i.e., have causal properties;
- How Claude Bernard, Walter Cannon, and Norbert Weiner established the importance of negative feedback for maintaining stability within large systems, leading to subsequent demonstration of negative feedback at the molecular level;
- How the identification of feed-forward mechanisms led to advances in understanding the features characterizing the design of systems control mechanisms.
Growth of Publications Relating Systems Biology
The historical pace of research in systems biology greatly accelerated at the beginning of the 21st century. The figure below shows the exponential growth of publications relating to or discussing systems biology in the decade 1996-2006. The data points derive from the National Library of Medicine's PubMed database, and likely underestimate annual "systems biology" publication rates by not including articles in journals in many non-biological disciplines that apply to the subject.
Early Systems Modelers
In 1952, the British neurophysiologists and nobel prize winners Alan Lloyd Hodgkin and Andrew Fielding Huxley constructed a mathematical model of the action potential - the fundamental mechanism underlying communication between nerve cells, expressing it as the consequence of a dynamic interaction between interdependent ionic conductances of the cell membrane. In 1960, Denis Noble developed the first computer model of a beating heart. Systems biologists invoke these pioneering pieces of work as illustrative of the systems biology project.
Systems Biology As a Reflection and a Transmutation of Classical Integrative Physiology
In the view of physiologists and bioengineers Daniel A. Beard and Marko Vendelin, the term 'systems biology' and 'physiology' synonymize, as he argues:
- "In a nutshell, "systems biology" attempts to realize this [its] promise by measuring and mapping biological interactions within cells, tissues, organs, and organ systems, and predicting how integrated systems made up of many interacting components behave. Readers of the American Journal of Physiology may wish to point out that by such a definition, the term "systems biology" is essentially a synonym for "physiology." While we would not argue with that assessment, we suggest that the increasing attention being paid to the endeavor is at least in part due to the injection of a new term, and associated new techniques and technologies, into the well-established field of integrative physiology. It has been argued that this increase in attention places the discipline of physiology in a position where it will either be "superseded...by systems biology" or will enjoy a renaissance as it leads the way in research in this area..."[8]
Molecular physiologist and biophysicist Kevin Strange earlier made the comparison:
- ”Physiology and systems biology share the goal of understanding the integrated function of complex, multicomponent biological systems ranging from interacting proteins that carry out specific tasks to whole organisms."[9]
In writing of systems biology, Strange encourages 21st century physiology departments “to embrace them as essential components” of their field, by ‘them’ referring specifically to “functional genomics, nonmammalian model organisms and computational biology”. He sees a ‘critical mass’-type advantage to a diverse group of physiologists and post-genomics-brand systems biologists working as a group.
Many others have likened physiology and systems biology. Certainly physiology dates to the beginnings of biology as a discipline, and systems biology dates even to Aristotle. Perhaps someone will write an integrated history of the 'History of Physiology' and 'History of Systems Biology'. Constraints on prolixity discourage further development of issues pertaining to the relation of physiology and systems biology.
Modeling in Systems Biology
For systems biologists, modeling serves as the key to unlocking the system of interest, whether a cell, a species, or an ecosystem.
Reminding us of the story of the six blind men investigating a different part of an elephant and coming up with six different descriptions of the animal, James Haefner[10] wrote:
- One failure of the blind men was to ignore the relations between objects. A seventh man, one sensitive to the importance of testing alternative models, might have said: “Hmmm, ‘tree’, ‘snake’, ‘fan’, ‘spear’, ‘wall’, ‘rope’: It’s a single, big thing with columnar supports and appendages at the ends.” The blind men, especially, need a systems approach, and with respect to the scientific unknown, we are all blind.
To understand a key systems approach, one must know something about model building, or simply, modeling, especially computer-based quantitative modeling. Models represent (re-present) reality in an abstract form, such as a diagram, a rule, a theory, a graph, a formula, a set of equations, a computer program. Systems biologists use numerous different quantitative mathematical models as keys to understanding biological systems—their logic, say—and as keys to predicting and controlling their behavior.
Quantitative models of biological systems potentially serve these main functions:
- They allow management of data sets of structures and interactions too large and complex for the human mind to manage without the “exo-cortex” computer-based quantitative representation;
- They participate in an iterative process whereby experimental data inspires a model and the model suggests the need for further experimental research the results of which inspire changes in the model, which repeats the cycle;
- By failing to fully account for the targeted behavior of the system:
- they raise the possibility of the existence of unknown subsystems of the system that require further experimental work, and may give direction to that further research;
- they inspire refinements of the model to account for the gaps;
- They provide an satisfying and usable ‘explanation’ of the system—an explanation of its properties, functions and behaviors in quantitative terms of its coordinated, dynamically interacting, hierarchically arranged components;
- They enable ‘control’ of the system—the ability to induce a desired behavior or propensity with the appropriate manipulation;
- They enable ‘prediction’ of the behavior of the system—predicting how it will respond to a given set of circumstances, especially predicting novel behaviors of the system.
Despite the large datasets available for many biological systems, especially cell systems, they still fall short in quantity and quality for realizing the full potential of quantitative modeling in applying advanced methodologies for statistical analyses, testing of hypotheses, and estimating the values of equation constants and independent variables.[11] Wellstead et al.[11] note the “enormous challenges to bio-sensing for systems biology” that lie ahead.
Some Examples of Familiar Models
The examples below facilitate learning about modeling heuristically.
A road map of an urban complex: For a given level of detail, a road map represents (re-presents, or models) the structure of the system of roads in a region of land at a given point in time, using the language of graphic illustration. In network parlance, cities and other points of interest (e.g., parks, lakes) serve as nodes and the connections between nodes serve as edges, some of which may indicate one-way connections only. Using the road map, one can control the system of roads in the sense that one can exploit the information to get from one place (node) to another, and predict how long it will take to get there. The level of detail may not allow determination of the grades of the roads (steepness) or the degrees of curviness or the position of side roads or new roads added since the date of the map.
Systems biologists use maps, for example, to model the structure and interrelationships among biochemical molecules in cellular subsystems, such as the subsystems that convert glucose to usable energy and to other biochemical molecules (e.g., glycogen).
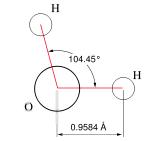
A chemical formula: Anyone reading this far knows H2O as a model (representation) for the substance, water. As such it has many uses, in particular in modeling chemical reactions. Other models of water, such as HOH, or
provide additional information for particular purposes.
A computer-based flight simulator: For a given level of detail, a computer-based flight simulator represents the multi-subsystem of a flight vehicle system (e.g., airplane), including its control characteristics, and the environment in which the flight vehicle must interact with in order to function stably and exhibit its system behavior of taking off, flying to destination, and landing. The representation (model) uses the languages of engineering, mathematics, computer programs, and dynamical graphics, among others to enable control of the system and to predict its behavior in response to a given set of environmental conditions for comparison with the response to those conditions in real flight—testing the goodness of fit of the model.
Systems biologists use computer-based simulators, for example, to predict the behavior of the human heart in response to drugs.[12]
Newton’s equations: For macroscopic mechanical systems, Newton’s equations represent, or model, the behavior of bodies of mass in motion, allowing predictions of the trajectories of moving masses, including the masses comprising the solar system; allowing calculation of the strength of the mutual attraction of masses and the contribution of each of two masses to that attraction; enabling explanation of macroscopic properties of gases and liquids (e.g., pressure, temperature) from the average effects of microscopic particles interacting in conformity with Newton’s equations; and, enabling engineers to construct vehicles for transporting humans to the moon and back.
(Erwin Schrodinger's wave equation does for subatomic mechanics what Newton's equations did for macroscopic mechanics, and Albert Einstein's General Relativity theory explained away Newton's action at a distance--as models advance in capability.)
Systems biologists use mathematical expressions, often more complex than Newton’s equations, based on empirical data and theoretical principles, in numerous ways to model the behavior of biological systems at all hierarchical levels.[13] See below.
Examples of Modeling in Systems Biology
Introductory remarks here…
Darwin’s theory of evolution by means of natural and sexual selection: Darwin’s theory, stated conceptually in words, and its 20th century amplification, stated genetically/molecularly often in quantitative terms, represents, or models, the behavior of nature in creating species and varieties sufficiently adapted to their environment to survive long enough to reproduce and care for their offspring and kin. The theory enables understanding of an enormous range of behaviors of animals, plants, unicellular organisms, and cellular and subcellular systems, and has explanatory and predictive value in every biological discipline, and many non-biological disciplines.
Evolutionary principles permeate the discipline of systems biology and have led to the emerging discipline of “evolutionary system biology”[14] A case in point: Determining whether natural selection operating at the molecular level has forged the structure of molecular networks may enhance understanding of their underlying design principles and thereby facilitate design of better predictive models.[15]
Bipedal and Quadrupedal Walking and Running: In the human organism, walking and running emerge as system behaviors (no subsystem itself walks or runs). The energy cost to the system of those locomotor behaviors defines a property of the system applicable to those behaviors. The energy cost owes to the appropriate forces the system must generate to support itself against gravity and to swing the locomoting limbs to achieve forward motion. Researchers have found that the rate at which the system produces those forces—shorthanded simply to ‘force production’—correlates with the system’s energy cost of locomotion. Thus, if one could develop a mathematical model that predicts force production from readily determined values of variables related to anatomy (e.g., limb length) and motion (e.g., forward speed), that model could then predict the system property of energy cost of locomotion.
Based on the findings of earlier studies, Harvard anthropologist Herman Pontzer[16] developed a mathematical model—viz., an equation—that justified force production as a function of three variables: the rate of muscular force production in the vertical direction, the rate of muscular force production in the horizontal direction, and the rate of muscular force production required to swing the limbs. From empirical data, knowledge of trigonometry and physics (force mechanics) and of muscle physiology, he identified measureable anatomical and motor variables--length and proportion of limbs, speed, frequency of stride, and angle of excursion--that allowed estimation of those required three force variables. Following earlier studies that linked force production with cost of locomotion, he generated the model--the equation--that he hoped would predict the latter from the former. He found that the model well predicted the observed cost of locomotion, somewhat better for running than walking.
Subsequently, Professor Pontzer tested the model in quadrupeds as well as humans.[17] The model proved superior to previous models, extending it to quadrupeds, and confirming the predictive ability of considering the proposed anatomical variables in estimating the rate of force production and energy cost of locomotion.
With the development of quadruped and biped robots for human service, Professor Pontzer's model might help make decisions on energy-cost-effective robot locomotor anatomy.
Modeling by Engineering Synthetic Systems: Systems biologists model systems also by using advanced and innovative experimental techniques to construct synthetic versions of a system with real system elements, often inserted in the larger system embedding the real system. They propose a design blueprint for the synthetic system based on empirical data about the system and the results of mathematical and computational tools used to analyze that data. With appropriate designs or tags, they can then observe and analyze the behavior of the synthetic system, in effect viewing it in isolation from the larger system embedding it. That potentially leads to insights in ways of manipulating the system for desired ends, including inducing novel behaviors of the system and the larger system embedding it.[18]
[...specific examples of synthetic systems here...]
Multi-Level Modeling of the Heart: [...describe work of Denis Noble and colleagues..]
[...Work in progress…More examples to come...]
Examples of Applications of Systems Biology Research
Modeling the Mitochondrion
Vo and Palsson[19] reviewed the advances systems biology research has made in explaining the integrated workings of the complex cellular subsystem, the mitochondrion—the cell’s transformer of energy taken into the cell to a cellular energy-currency:
- ”With the rapidly increasing number of discovered molecular components [of mitochondria], computational models are also being developed to facilitate the organization and analysis of such data. Computational models of mitochondria have been accomplished with top-down [starting with a theoretical model of the system then tested against the data] and bottom-up [starting with the data and building up to the model] approaches and have been steadily improved in size and scope. Results from top-down methods tend to be more qualitative but are unbiased by prior knowledge about the system. Bottom-up methods often require the incorporation of a large amount of existing data but provide more rigorous and quantitative information, which can be used as hypotheses for subsequent experimental studies.”
To cite one example: Using massive data sets on mitochondrial components and chemical reactions, combined with mathematical assumptions and computer-based mathematical analyse, Vo et al.[20] ‘constructed’ (aka modeled) a network of biochemical pathways that preserved the known interactions among the components. The constructed networked described mitochondrial energy production and other mitochondrial functions closely in accord with experimental results.
The authors recognized the need for more experimental data of mitochondrial components and function to refine the model for predictability.
Emergent Properties as ‘Materialistic Vitalism’
Cell biologists might find it tempting to see a type of 'vitalism' in living systems, in view of the concept that some high-level features of organisms, perhaps including even activity of living itself, exemplify emergent phenomena---phenomena not explainable by studying each of the chemical processes that occur in the cell in separation from its integration in the cell system embedding it.[21] Because biologists and their co-scientists can explain emergent properties/phenomena in principle by mechanisms not disconnected from interactions of matter and energy, any such ‘vitalism’ properly qualifies only as ‘materialistic vitalism’.
For example of emergence, when individual chemical processes form interconnected feedback cycles which produce products perpetuating those cycles rather than unconnected products, they can form systems with properties that the reactions, taken individually, lack.[22].
For another example, in studying a protein separated from the system it belongs to, one can observe many of its properties, but in so studying the protein one cannot observe any of the properties it has only in the context of the system that embeds it, such as the property of catalyzing a biochemical reaction, or of binding to other proteins to form a functional protein complex. Those properties of the protein emerge in the context of the protein’s environment. Moreover, those emergent properties may result in effects within the system that in a feedback way further alters the properties of the protein in the system, as when a reaction product alters the catalytic properties of the protein.
Every biological system (e.g., a biochemical pathway) resides in a larger, more complex 'parent' system (e.g., an interconnected cycle with feedback)---essentially its environment---that has effects on the 'child' system. Those effects in turn can change the properties and behaviors of the 'child' system's subsystems that would not have existed without the system in its environment---a kind of 'downward causation'. Thus, in an environment of other birds of a feather, a system with flocking behavior may form.
As Gilbert and Sarkar[22] puts it: “Thus, when we try to explain how the whole system behaves, we have to talk about the context of the whole and cannot get away talking only about the parts.”
Emergent processes have been recognised as, for example, contributing to understanding:
- subcellular morphology [23],
- developmental biology [24],
- metabolic networks [25],
- proteomics [26]
Emergent phenomena appear even in non-biological physical systems [27]. Emergent phenomena attract the attention of cellular neuroscientists; [28] and cognitive scientists [29]. At still higher systems levels, emergent properties appear for example in the behaviour of ant colonies and the concept of swarm intelligence, [30] Artificial systems scientists have simulated emergent phenomena [31]
Emergent phenomena in human societies has also received attention. [32].
Biologists can also study the biosphere itself as emergent. [33]
References
- ↑ Property: quality or trait peculiar to a thing (e.g., mass, volume, ability to reproduce, structure, lifespan, etc.; function: action specially fitted for a thing (e.g., locomotion, phagocytosis, phototropism, functioning as a molecular motor, energy transduction, etc.); behavior: the activity detected by the observer (e.g., deception, flight, chemotaxis, etc.). The distinctions among those often blur, ‘property’ serving generically in many instances.
- ↑ Kitano H (2002) Systems biology: a brief overview Science 295:1662-1664 PMID 11872829
- ↑ Keller EF (2005) The century beyond the gene. J Biosci 30:3-10 PMID 15824435
- ↑ 4.0 4.1 Westerhoff HV, Palsson BO (2004) The evolution of molecular biology into systems biology Nature Biotechnology 22:1249-52
- ↑ Westerhoff HV and Alberghina L (2005), 'Systems Biology: Did we know it all along?', in Topics in Current Genetics, Vol. 13: Systems Biology, ed. Alberghina L and Westerhoff HV, pp.3-9. Berlin: Springer-Verlag. ISBN 978-3-540-22968-1
- ↑ Trewavas AJ (2006), A Brief History of Systems Biology: "Every object that biology studies is a system of systems." Francois Jacob (1974). Plant Cell 18:2420-30 PMID: 17088606
- ↑ Aristotle On Causality.The Stanford Encyclopedia of Philosophy
- ↑ Beard DA, Vendelin M. (2006) Systems biology of the mitochondrion. Am J Physiol Cell Physiol 291:C1101-C1103 PMID 17102034 Full-Text
- ↑ Strange K. The end of "naive reductionism": rise of systems biology or renaissance of physiology? Am J Physiol Cell Physiol 2005;288:C968-C974 PMID 15840560 Full-Text
- ↑ Haefner JW. (2005) Modeling Biological Systems: Principles and Applications. Springer, ISBN 0387250115
- ↑ 11.0 11.1 Wellstead P, Middleton R, Wolkenhauer O (2006) Feedback Medicine: Control Systems Concepts in Personalised, Predictive Medicine and Combinatorial Intervention. Follow link to full-text
- ↑ Noble D (2006) “Multilevel Modeling in Systems Biology: From Cells to Whole Organs”. In, Szallasi Z, Stelling J, and Periwal V. (editors) (2006) System Modeling in Cell Biology From Concepts to Nuts and Bolts. A Bradford Book, MIT Press, Cambridge, MA ISBN 0262195488 Chapter 14, pp. 297-312
- ↑ Szallasi Z, Stelling J, and Periwal V. (editors) (2006) System Modeling in Cell Biology From Concepts to Nuts and Bolts. A Bradford Book, MIT Press, Cambridge, MA ISBN 0262195488
- ↑ Medina M (2005) Genomes, phylogeny, and evolutionary systems biology PNAS 102:6630-5 PMID 15851668
- ↑ Wagner A (2003)Does Selection Mold Molecular Networks? Sci.STKE 2003:e41 PMID 14519859
- ↑ Pontzer H. (2005) A new model predicting locomotor cost from limb length via force production. J Exp Biol 208:1513-24
- ↑ Pontzer H. (2007) Predicting the energy cost of terrestrial locomotion: a test of the LiMb model in humans and quadrupeds. J Exp Biol 210:484-94 PMID 17234618
- ↑ Hasty J, McMillen D, Collins JJ (2002) Engineered gene circuits. Nature 420:224-30
- ↑ Vo TD, Palsson BO. (2007) Building the power house: recent advances in mitochondrial studies through proteomics and systems biology. Am J Physiol Cell Physiol 292:C164-C177 PMID 16885397 Full-Text
- ↑ Vo TD, Greenberg HJ, Palsson BO. Reconstruction and Functional Characterization of the Human Mitochondrial Metabolic Network Based on Proteomic and Biochemical Data. J.Biol.Chem. 2004;279:39532-40 PMID 15205464 Full-Text
- ↑ Berg EL et al (2005) Biological complexity and drug discovery: a practical systems biology approach Syst Biol 152:201-6 PMID 16986261
- ↑ 22.0 22.1 Gilbert SF, Sarkar S (2000) Embracing complexity: organicism for the 21st century Dev Dyn 219:1-9 PMID 10974666
- ↑ Tabony J (2006) Microtubules viewed as molecular ant colonies Biol Cell 98:603-17 PMID 16968217
- ↑ e.g. Theise ND, d'Inverno M (2004) Understanding cell lineages as complex adaptive systems Blood Cells Mol Dis 32:17-20 PMID 14757407 and Ruiz i Altaba A, et al (2003) The emergent design of the neural tube: prepattern, SHH morphogen and GLI code Curr Opin Genet Dev 13:513-21 PMID 14550418
- ↑ Jeong H et al(2000) The large scale organisation of metabolic networks Nature 407:651-4 [1]
- ↑ e.g. Grindrod P, Kibble M (2004) Review of uses of network and graph theory concepts within proteomics Expert Rev Proteomics 1:229-38 PMID 15966817 and Ye X, Chu J, Zhuang Y, Zhang S (2005) Multi-scale methodology: a key to deciphering systems biology Front Biosci 10:961-5 PMID 15569634
- ↑ Cho YS et al (2005) Self-organization of bidisperse colloids in water droplets J Am Chem Soc 127:15968-75 PMID 16277541
- ↑ see e.g. Burak Y, Fiete I (2006) Do we understand the emergent dynamics of grid cell activity? J Neurosci 26:9352-4 PMID 16977716
- ↑ e.g. Courtney SM (2004) Attention and cognitive control as emergent properties of information representation in working memory Cogn Affect Behav Neurosci 4:501-16 PMID 15849893
- ↑ Theraulaz G et al (2002) Spatial patterns in ant colonies Proc Natl Acad Sci USA 99:9645-9 PMID 12114538
- ↑ Theraulaz G, Bonabeau E (1999)A brief history of stigmergy Artif Life 5:97-116 PMID 10633572
- ↑ Bonabeau E, Meyer C (2001) Swarm intelligence. A whole new way to think about business Harv Bus Rev 79:106-14 PMID 11345907
- ↑ Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. (1998) Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 281:237-40 [http://www.sciencemag.org/cgi/content/full/281/5374/237/ Full-Text
Further Reading, Notes and Links
Books
- Alon U. (2007) An Introduction to Systems Biology: Design Principles of Biological Circuits. Boca Raton: Chapman and Hall/CRC ISBN 1-58488-642-0
- 301 pages; 12 chapters; 4 appendixes; 23 pages of references; glossary; Table of Contents
- Kaneko K. (2006) Life: An Introduction to Complex Systems Biology. Berlin: Springer ISBN 3-540-32666-9
- 370 pages; 12 chapters; 127 figures; 16 pages of references; Table of Contents
- Palsson B. (2006) Systems Biology - Properties of Reconstructed Networks. Cambridge University Press ISBN 9780521859035
- Szallasi Z, Stelling J, Periwal V (eds). (2006) System Modelling in Cellular Biology: From Concept to Nuts and Bolt. A Bradford Book, The MIT Press ISBN 0-262-19548-8
- 480 pages; 17 Chapters; 36 Contributors; Table of Contents and Full-Texts: Preface; Sample Chapter; Contributors; References
- Schneider ED, Sagan D. (2005) Into the Cool: Energy Flow, Thermodynamics, and Life. Chicago: The University of Chicago Press ISBN 0-226-73937-6
- Klipp E, Herwig R, Kowald A, Wierling C, Lehrach H. (2005) Systems Biology in Practice. Wiley-VCH: ISBN 3-527-31078-9
- Bock G and Goode JA (eds). (2002) In Silico" Simulation of Biological Processes, Novartis Foundation Symposium 247. John Wiley & Sons ISBN 0-470-84480-9
- Kitano H (editor). (2001) Foundations of Systems Biology. MIT Press ISBN 0-262-11266-3