Carnot cycle: Difference between revisions
imported>Paul Wormer |
imported>Paul Wormer No edit summary |
||
| Line 1: | Line 1: | ||
{{Image|Carnot title page.jpg|right|275px|''Reflections on the motive power of fire and on the machines fitted to develop that power'' by S. Carnot alumnus of the École Polytechnique.}} | {{Image|Carnot title page.jpg|right|275px|''Reflections on the motive power of fire and on the machines fitted to develop that power'' by S. Carnot alumnus of the École Polytechnique.}} | ||
A '''Carnot cycle''' is a reversible cycle in the state space of a [[thermodynamics|thermodynamic system]]. The cycle consists of two [[isotherms]] (paths of constant [[temperature]]) and two [[isentropes]] (paths of constant [[entropy (thermodynamics)|entropy]]). | A '''Carnot cycle''' is a reversible cycle in the state space of a [[thermodynamics|thermodynamic system]] consisting of a cylinder filled with a substance that can absorb heat and deliver work and conversely. The cylinder and its content undergo a thermodynamic cycle that consists of two [[isotherms]] (paths of constant [[temperature]]) and two [[isentropes]] (paths of constant [[entropy (thermodynamics)|entropy]]). A heat engine that undergoes periodic Carnot cycles is a '''Carnot engine'''; it converts [[heat]] into [[work]]. A Carnot engine is an idealization of real engines, in actual practice it does not exist. | ||
A heat engine receives an amount of heat (thermal energy) ''Q''<sub>h</sub> from a heat source and delivers an amount of work (mechanical energy) ''W'' to its surroundings. Its efficiency is the ratio η ≡ ''W''/''Q''<sub>h</sub>. The two most important characteristics of the Carnot heat engine are: (i) η is independent of the substance inside the cylinder, and (ii) the Carnot cycle is the most efficient cycle possible. Other thermodynamic cycles, reversible or irreversible, deliver less work for a given amount of heat. | |||
==History== | ==History== | ||
In the early 1820s [[Sadi Carnot]], studying the efficiency of [[steam engine]]s, conceived a virtual, idealized, version of a steam engine as a vehicle for his ''Gedankenexperimente'' (thought experiments) in which he mimicked the work of actual steam engines. He reported the results of his studies in a booklet (see the adjacent title page) published in 1824.<ref>''Reflexions on the Motive Power of Fire'', translated and edited by R. Fox, Manchester University Press, (1986) [http://books.google.nl/books?id=tVzSAAAAIAAJ&printsec=frontcover&source=gbs_v2_summary_r&cad=0#v=onepage&q=&f=false Google books]</ref> By means of his engine Carnot tried to find answers to questions as: Would it be advantageous to use steam at high pressures, and if so, would there be a limit at which further raising of pressure ceases to be advantageous? How important is the working medium, is steam the best substance, or could any other liquids and their vapors, or air, perform as well? | In the early 1820s [[Sadi Carnot]], studying the efficiency of [[steam engine]]s, conceived a virtual, idealized, version of a steam engine as a vehicle for his ''Gedankenexperimente'' (thought experiments) in which he mimicked the work of actual steam engines. He reported the results of his studies in a booklet (see the adjacent title page) published in 1824.<ref>''Reflexions on the Motive Power of Fire'', translated and edited by R. Fox, Manchester University Press, (1986) [http://books.google.nl/books?id=tVzSAAAAIAAJ&printsec=frontcover&source=gbs_v2_summary_r&cad=0#v=onepage&q=&f=false Google books]</ref> By means of his engine Carnot tried to find answers to questions as: Would it be advantageous to use steam at high pressures, and if so, would there be a limit at which further raising of pressure ceases to be advantageous? How important is the working medium, is steam the best substance, or could any other liquids and their vapors, or air, perform as well? | ||
| Line 6: | Line 8: | ||
Conceptually, a steam engine is a very simple devise; its rudimentary form consists of a piston that cyclically moves in and out a cylinder, driven by expanding steam. The steam flows from the boiler (hot) to the condenser (cold). Inspired by this construction, Carnot devised an engine, basically a fluid-filled cylinder with a piston, that is alternately exchanging heat with a hot and a cold reservoir. When in contact with the hot reservoir, the engine receives heat and performs work on its surroundings—this stage is comparable to the steam engine piston moving out of the cylinder and performing work. When the cylinder is in contact with the cold reservoir, the surroundings perform work on the engine, i.e., compress the substance inside the cylinder, and the resulting heat is given off to the cold reservoir—this stage is comparable with the piston of a steam engine driven back into the cylinder. While this alternating process is going on, the thermodynamic state of a fixed amount of fluid inside the engine changes periodically, that is, the substance goes through thermodynamic cycles where after each cycle the thermodynamic state is exactly equal to the initial state (has the same temperature, pressure, volume, …). Carnot proved that it is irrelevant for his abstract engine what the actually working substance is, it may be water, steam, an ideal gas, or anything else. | Conceptually, a steam engine is a very simple devise; its rudimentary form consists of a piston that cyclically moves in and out a cylinder, driven by expanding steam. The steam flows from the boiler (hot) to the condenser (cold). Inspired by this construction, Carnot devised an engine, basically a fluid-filled cylinder with a piston, that is alternately exchanging heat with a hot and a cold reservoir. When in contact with the hot reservoir, the engine receives heat and performs work on its surroundings—this stage is comparable to the steam engine piston moving out of the cylinder and performing work. When the cylinder is in contact with the cold reservoir, the surroundings perform work on the engine, i.e., compress the substance inside the cylinder, and the resulting heat is given off to the cold reservoir—this stage is comparable with the piston of a steam engine driven back into the cylinder. While this alternating process is going on, the thermodynamic state of a fixed amount of fluid inside the engine changes periodically, that is, the substance goes through thermodynamic cycles where after each cycle the thermodynamic state is exactly equal to the initial state (has the same temperature, pressure, volume, …). Carnot proved that it is irrelevant for his abstract engine what the actually working substance is, it may be water, steam, an ideal gas, or anything else. | ||
At the time of writing his booklet, Carnot saw heat as a fluid, called "caloric", that "falls" from high to low temperature. He thought that the "motive power" that the caloric received from the hot reservoir was converted into work; the caloric itself was conserved and flowed back into the cold reservoir. Carnot was inspired by the equivalence of his engine with a water wheel that, in principle, can extract work from the potential energy ("motive power") of water that is initially | At the time of writing his booklet, Carnot saw heat as a fluid, called "caloric", that "falls" from high to low temperature. He thought that the "motive power" that the caloric received from the hot reservoir was converted into work; the caloric itself was conserved and flowed back into the cold reservoir. Carnot was inspired by the equivalence of his engine with a water wheel that, in principle, can extract work from the potential energy ("motive power") of water that is initially stored in a reservoir above the water wheel. Of course, in a water wheel the water itself does not disappear, it is conserved. Caloric may be equated to heat—a form of energy—and motive power is also a form of energy, therefore Carnot's ideas are contrary to conservation of energy—the [[first law of thermodynamics]]. Although the first law was not known to Carnot (and it was not even formulated during his life time), Carnot came very close to formulating the [[second law of thermodynamics]], especially in its form given by Thomson. For this reason it can be stated that the second law was discovered before the first law. | ||
In the 1850s, [[Rudolf Clausius|R. Clausius]] and [[Lord Kelvin|W. Thomson]] | In the 1850s, [[Rudolf Clausius|R. Clausius]] and [[Lord Kelvin|W. Thomson]] realized that the Carnot engine converts only part of the heat into work. A sizable amount of remaining heat is given off to the cold reservoir and the first law states that the remaining heat plus delivered work are equal to the input heat. Although Carnot's ideas were in contradiction to the first law, they proved to be very useful to Clausius and Thomson, nevertheless, when they postulated the second law of thermodynamics. | ||
==Definition== | ==Definition== | ||
| Line 19: | Line 21: | ||
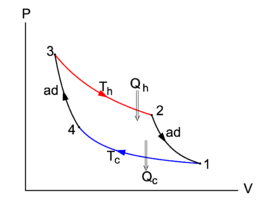
{{Image|Carnot cycle.png|right|275px|Fig. 1. <small>The Carnot cycle in a ''P-V'' diagram. The area enclosed by the closed curve is the net work performed by the system (arrows clockwise)</small>}} | {{Image|Carnot cycle.png|right|275px|Fig. 1. <small>The Carnot cycle in a ''P-V'' diagram. The area enclosed by the closed curve is the net work performed by the system (arrows clockwise)</small>}} | ||
In Figure 1 the Carnot cycle is represented in | In Figure 1 the Carnot cycle is represented in a ''P-V'' diagram, as was first done by [[Clapeyron]] in 1834. Consider the compression (decreasing volume) of the gas along the path 1–4. Along this path the cylinder is in thermal equilibrium with the cold reservoir; both have temperature ''T''<sub>c</sub>. The environment acts on the piston, moving it slowly (in order to keep the process reversible) into the cylinder; the work done is converted into heat ''Q''<sub>c</sub> that is given off to the cold reservoir. This process is so slow that the temperature remains constant (i.e., it is an isothermic process), and hence the internal energy ''U'' of the gas does not change, ''dU'' = 0. By the first and second law of thermodynamics, reference to Figure 1, and the ideal gas law ''PV'' = ''RT'' (for the sake of argument we consider one mole of gas): | ||
:<math> | :<math> | ||
Q_c = T_c \int_1^4 dS = \int_1^4 P dV = RT_c \int_1^4 \frac{dV}{V} \Longrightarrow | Q_c = T_c \int_1^4 dS = \int_1^4 P dV = RT_c \int_1^4 \frac{dV}{V} \Longrightarrow | ||
Revision as of 04:53, 15 November 2009
A Carnot cycle is a reversible cycle in the state space of a thermodynamic system consisting of a cylinder filled with a substance that can absorb heat and deliver work and conversely. The cylinder and its content undergo a thermodynamic cycle that consists of two isotherms (paths of constant temperature) and two isentropes (paths of constant entropy). A heat engine that undergoes periodic Carnot cycles is a Carnot engine; it converts heat into work. A Carnot engine is an idealization of real engines, in actual practice it does not exist.
A heat engine receives an amount of heat (thermal energy) Qh from a heat source and delivers an amount of work (mechanical energy) W to its surroundings. Its efficiency is the ratio η ≡ W/Qh. The two most important characteristics of the Carnot heat engine are: (i) η is independent of the substance inside the cylinder, and (ii) the Carnot cycle is the most efficient cycle possible. Other thermodynamic cycles, reversible or irreversible, deliver less work for a given amount of heat.
History
In the early 1820s Sadi Carnot, studying the efficiency of steam engines, conceived a virtual, idealized, version of a steam engine as a vehicle for his Gedankenexperimente (thought experiments) in which he mimicked the work of actual steam engines. He reported the results of his studies in a booklet (see the adjacent title page) published in 1824.[1] By means of his engine Carnot tried to find answers to questions as: Would it be advantageous to use steam at high pressures, and if so, would there be a limit at which further raising of pressure ceases to be advantageous? How important is the working medium, is steam the best substance, or could any other liquids and their vapors, or air, perform as well?
Conceptually, a steam engine is a very simple devise; its rudimentary form consists of a piston that cyclically moves in and out a cylinder, driven by expanding steam. The steam flows from the boiler (hot) to the condenser (cold). Inspired by this construction, Carnot devised an engine, basically a fluid-filled cylinder with a piston, that is alternately exchanging heat with a hot and a cold reservoir. When in contact with the hot reservoir, the engine receives heat and performs work on its surroundings—this stage is comparable to the steam engine piston moving out of the cylinder and performing work. When the cylinder is in contact with the cold reservoir, the surroundings perform work on the engine, i.e., compress the substance inside the cylinder, and the resulting heat is given off to the cold reservoir—this stage is comparable with the piston of a steam engine driven back into the cylinder. While this alternating process is going on, the thermodynamic state of a fixed amount of fluid inside the engine changes periodically, that is, the substance goes through thermodynamic cycles where after each cycle the thermodynamic state is exactly equal to the initial state (has the same temperature, pressure, volume, …). Carnot proved that it is irrelevant for his abstract engine what the actually working substance is, it may be water, steam, an ideal gas, or anything else.
At the time of writing his booklet, Carnot saw heat as a fluid, called "caloric", that "falls" from high to low temperature. He thought that the "motive power" that the caloric received from the hot reservoir was converted into work; the caloric itself was conserved and flowed back into the cold reservoir. Carnot was inspired by the equivalence of his engine with a water wheel that, in principle, can extract work from the potential energy ("motive power") of water that is initially stored in a reservoir above the water wheel. Of course, in a water wheel the water itself does not disappear, it is conserved. Caloric may be equated to heat—a form of energy—and motive power is also a form of energy, therefore Carnot's ideas are contrary to conservation of energy—the first law of thermodynamics. Although the first law was not known to Carnot (and it was not even formulated during his life time), Carnot came very close to formulating the second law of thermodynamics, especially in its form given by Thomson. For this reason it can be stated that the second law was discovered before the first law.
In the 1850s, R. Clausius and W. Thomson realized that the Carnot engine converts only part of the heat into work. A sizable amount of remaining heat is given off to the cold reservoir and the first law states that the remaining heat plus delivered work are equal to the input heat. Although Carnot's ideas were in contradiction to the first law, they proved to be very useful to Clausius and Thomson, nevertheless, when they postulated the second law of thermodynamics.
Definition
Carnot depicted his engine as an upright cylinder, containing a fluid, with adiabatic walls and its top covered by a movable piston. The bottom of the cylinder can be changed from adiabatic to heat conducting. The cylinder is placed alternately on top of a cold heat reservoir (of absolute temperature Tc) and on a hot reservoir (of temperature Th). It will be proved below that the nature of the fluid is irrelevant for the thermodynamics of the process, so that it is most convenient to assume that the fluid is an ideal gas. By the equipartition theorem, the internal energy of one mole of an ideal gas is,
where f, the number of degrees of freedom, is 3 for a mono-atomic gas, 5 for a diatomic gas, and 6 for a gas of arbitrarily shaped molecules. The quantity R is the molar gas constant. For the present discussion it is important to notice that U is a function of T only.
In Figure 1 the Carnot cycle is represented in a P-V diagram, as was first done by Clapeyron in 1834. Consider the compression (decreasing volume) of the gas along the path 1–4. Along this path the cylinder is in thermal equilibrium with the cold reservoir; both have temperature Tc. The environment acts on the piston, moving it slowly (in order to keep the process reversible) into the cylinder; the work done is converted into heat Qc that is given off to the cold reservoir. This process is so slow that the temperature remains constant (i.e., it is an isothermic process), and hence the internal energy U of the gas does not change, dU = 0. By the first and second law of thermodynamics, reference to Figure 1, and the ideal gas law PV = RT (for the sake of argument we consider one mole of gas):
where log is the natural (base e) logarithm. The entropy of an ideal gas is a logarithmic function of its volume.
The isotherm 1–4 has the following functional form in the P-V plane,
At point 4 the cylinder is removed from the cold reservoir, its bottom is changed to adiabatic, so that the whole cylinder, including the piston, cannot exchange heat with the surroundings. The piston is moved inward from V4 to V3 and by this compression the gas is heated up to the temperature Th of the hot reservoir. In order that heat can be exchanged reversibly between the cylinder and the hot reservoir it is necessary that cylinder and reservoir have the same temperature. From 4 to 3 the path in the P-V diagram is adiabatic and reversible (isentropic). The entropy does not change during this compression, dS = 0. It is easy to derive the equation for the adiabatic in the P-V plane,
For a mono-atomic gas (f=3) the functional form of the curve 4-3 is:
The integral is the work done by the surroundings on the cylinder. In the P-V diagram it is the negative of the surface bounded by the curve 1–4–3 and the V axis.
At point 3 the bottom of the cylinder, with temperature Th, is changed back to heat-conducting, and the cylinder is placed on the top of the hot reservoir. Slowly the piston moves out, meaning that the cylinder performs work on the surroundings. While this happens the cylinder receives in total the heat Qh from the hot reservoir. The isotherm 3–2 has the functional form P(V) = RTh/V and the entropy increases:
From 2 to 1 isentropic expansion occurs, work is done by the cylinder on the surroundings, and its temperature decreases to the temperature Tc of the cold reservoir. The integral is the work done by the cylinder. In the P-V diagram it is the surface bounded by the curve 3–2–1 and the V axis. Note that this surface is larger than the surface below the curve 1–4–3. In total the work done during the cycle is difference of the the two surfaces:
- The total work done by the Carnot engine is the area enclosed by the cyclic path in the PV-diagram.
By the first law of thermodynamics, the total work W is equal to Qh − Qc, part of the heat Qh received from the hot reservoir is converted into work, part is passed on to the cold reservoir.
The process, as depicted in Figure 1, runs clockwise. All the processes are reversible, so that all arrows can be reverted. The integral over the path from 1 to 3 is then the negative of the area under this curve and represents the work done by the surroundings on the system (cylinder). The integral from 3 to 1 gives the work done by the system. In short, when the arrows run anti-clockwise, the area enclosed by the closed curve in Figure 1 is the work done by the surroundings on the system. This work results in a extraction of heat Qc = Qh − W from the cold reservoir, i.e., the engine works as a heat pump when it runs in anti-clockwise direction.
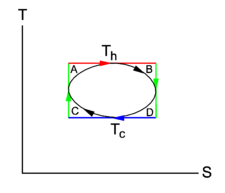
By definition it is reversible (quasi-static) and consists of the "hot" isotherm 2-3, the "cold" isotherm 1-4 and the adiabatics (isentropes) 1-2 and 3-4.
(To be continued)
- ↑ Reflexions on the Motive Power of Fire, translated and edited by R. Fox, Manchester University Press, (1986) Google books