Hepatitis B virus: Difference between revisions
imported>Robert Badgett |
imported>Robert Badgett |
||
| Line 115: | Line 115: | ||
==Prevention== | ==Prevention== | ||
The [[Centers for Disease Control and Prevention]] have issued recommendations for vaccination against hepatitis B among patients with diabetes.<ref>{{ | The [[Centers for Disease Control and Prevention]] have issued recommendations for vaccination against hepatitis B among patients with diabetes.<ref name="pmid22189894">{{cite journal| author=Centers for Disease Control and Prevention (CDC)| title=Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). | journal=MMWR Morb Mortal Wkly Rep | year= 2011 | volume= 60 | issue= 50 | pages= 1709-11 | pmid=22189894 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22189894 }} </ref> | ||
| title = Use of | |||
| | |||
| url = http://www. | |||
}}</ref> | |||
==Current research== | ==Current research== | ||
Revision as of 20:08, 2 February 2012
| Hepatitis B virus | ||||||
|---|---|---|---|---|---|---|
 | ||||||
| Virus classification | ||||||
|
Description and significance
Hepatitis B virus (HBV) is a member of the hepadnavirus family and causes serum hepatitis, meaning, inflammation of the liver.
Hepatitis B is one of the most common infectious diseases in the world. According to the Centers for Disease Control (CDC), an estimated 800,000–1.4 million people in the United States have chronic HBV infection. About 5,000 people die yearly from hepatitis-related cirrhosis and about 1,000 die from HBV-related liver cancer. This problem is even greater worldwide, approximately 350 million people are chronically infected. An estimated 620,000 people worldwide die from HBV-related liver disease each year.[1]
Depending on the patient’s immune response, infection by HBV can be asymptomatic, chronic or acute and may range from mild to severe. Many people infected with HBV never develop any symptoms and may not even know they carry the virus in their bodies; nevertheless, they are still able to pass the virus on to other people. Such people are said to be carriers of the disease.
Cell structure
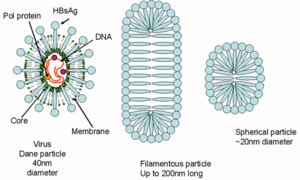
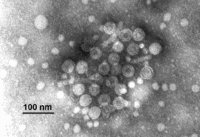
The hepatitis B virus is a complex virus with a central core and a protein coat. The virions of Hepatitis B virus, also known as Dane particles, are 42nm in diameter and have an isometric nucleocapsid core of 27nm in diameter. This core structure is surrounded by an outer shell that is approximately 4 nm in thickness. The protein of the virion coat is known as the "surface antigen", or HBsAg, that is sometimes extended as a tubular tail on one side of the virus particle. This antigen is commonly produced in vast excesses, and can be seen in the blood of infected individuals in the form of filamentous and spherical particles. Filamentous particles are identical to the virion tails in that they vary in length and have a mean diameter of about 22nm. They sometimes display regular, non-helical transverse striations.[2]
The glycoproteins on the virus surface contain antigenic determinants that are group specific and type specific. Using these determinants, epidemiologists identify eight subtypes of HBV, denoted as A through H. HBV genotypes show a characteristic geographical distribution:
Genotype A is pandemic but most prevalent in northern Europe, North America and central Africa.
Genotypes B and C are found in eastern Asia, Korea, China, Japan, Polynesia and Vietnam.
Genotype D is also pandemic but is predominant in the Mediterranean area, the Middle East and India.
Genotype E is typical for Africa.
Genotype F is found in American natives and in Polynesia.
Genotype G in Western Europe and North America.
Genotype H is found predominantly in Central America.
Genotyping of HBV is important not only for molecular epidemiology purposes. Several recent research studies demonstrated that the rate of the chronic outcome and the severity of liver disease can be different for some genotypes.[3]
Genome structure
HBV is a mostly double-stranded DNA virus classified as Orthohepadnavirus within the Hepadnaviridae family. HBV causes hepatitis in human and related viruses in this family cause hepatitis in ducks, ground squirrels and woodchucks.
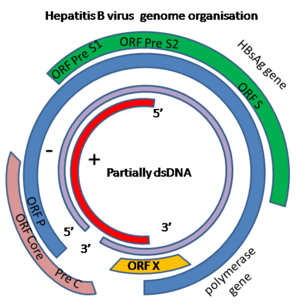
The genome is –RT, not segmented and contains a single circular molecule. It is partially double-stranded DNA that forms a covalently closed circle (with 5' end of the full length minus strand which is linked to the viral DNA polymerase). The complete genome is 3020-3320 nucleotides long, or 1700-2800 nucleotides long (for the full and short length strand, respectively). The genome has a guanine + cytosine content of 48 %. The genome sequence has termini with cohesive ends that match the uniquely located 5'-ends of the two strands which overlap by approximately 240 nucleotides and maintain the circular configuration of the DNA. The sequence has ENH an enhancer region and a direct repeat sequence (DR1 andDR2), or a U5-like sequence, a polyadenylation signal, and a putative glucocorticoid-responsive element; negative-sense or non-coding strand (complementary to the viral mRNA) is full-length (3.0-3.3 kb, positive sense strand (the viral mRNA) is variable in size and shorter than full-length. The double stranded genome has a nick at a unique site on full length negative strand opposite a position of 50 nucleotides, or 242 nucleotides downstream from the 5' end of the positive sense strand. The 5'-end of the negative-sense strand has a covalently attached terminal protein; positive-sense strand has a 5' capped oligoribonucleotide primer. [4]
The HBV genome has four genes: pol, env, pre-core and X that respectively encode the viral DNA-polymerase, envelope protein, pre-core protein (which is processed to viral capsid) and protein X. The specific function of protein X is not clear but it may be involved in the activation of host cell genes and the development of malignant cells.[5]
Ecology and Transmission
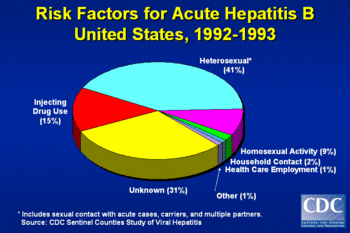
The virus is normally transmitted through contact with body fluids, usually blood contamination. However, the virus is also present in other fluids such as semen, vaginal fluids, menstrual blood, saliva and breast milk. Although injection of blood, as a result of intravenous drug use or the use of another blood contaminated instrument, is the most common route of infection, the virus can also be contracted via sexual intercourse (particularly male to male) and perinatally from the blood of an infected mother through the placenta to infect the fetus. Outside the body, HBV can survive at least 7 days and still be capable of causing infection.
According to the CDC approximately 78,000 people in the United States were infected by HBV in 2001 and about 5,000 people die per year from HBV-associated disease. One in 20 people in the United States is infected by HBV at some time in their lives with the highest infection rate being in young adults. About 5% of people infected by HBV get a chronic infection and there are more than one million Americans with chronic hepatitis B. Up to one quarter of these chronically-infected patients will die of some form of liver disease.
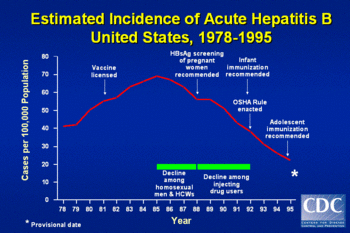
As a result of the currently available vaccine, the number of acute hepatitis B infections in the United States has been steadily decreasing. The rate of new HBV infections has declined by approximately 80% since 1991, when a national strategy to eliminate HBV infection was implemented in the United States. The decline has been greatest among children born since 1991, when routine vaccination of children was first recommended.[6]
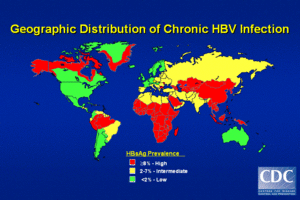
HBV is found all over the world. The highest prevalence of the HBV blood marker (HBsAg) is found in sub-Saharan Africa, the Far-East (China, Malaysia, Indonesia, Philippines, Papua New Guinea etc). Other locations that show high levels of HBV are in northern South America, northern Canada and Alaska and Greenland. In these areas, the lifetime risk of infection is greater than 60% with particularly common childhood infections. In areas of low prevalence of the virus, the lifetime risk of HBV infection is less than 20% with most infections occurring in adults who are in elevated risk groups. In the United States, less than 2% of the population present evidence of serum HBsAgbut, however, in Asian Americans chronic HBV may be as high as 10-15%. In Asian Americans, hepatocellular carcinoma is a leading cause of death. In fact, half of all children born to mothers with chronic HBV infection in America are Asian Americans. African Americans also show a high rate of HBV infection.
Replication
To infect a cell, HBV first attaches to the cell surface receptor of the host cell. To insert the viral genome into the cell, the viral membrane fuses with the cell membrane releasing the core into the cytoplasm. The core consists of proteins that are associated with the viral partially double stranded DNA. When the core enters the cytoplasm, the proteins dissociate from the DNA with the help of the virally-encoded polymerase (one of the core proteins, not the DNA polymerase that in the host nucleus). Next, the double stranded DNA enters the nucleus and its ends are ligated by the host enzymes, resulting in a viral circular episome. The viral DNA associates with host nuclear histones and is transcribed by cellular RNA polymerase II into mRNAs.
In contrast to retroviruses, the DNA of HBV is usually not integrated into the cellular DNA; rather it is found as an independent episome. This is because, unlike retroviruses, hepadnaviruses have no integrase activity. However, integrated parts of the HBV genome are found in the chromosomes of many hepatocellular carcinoma patients.
HBV's genome is composed of four genes, and therefore, four mRNAs are made from the HBV genome. To do that, the host cell polymerase interacts with four promoters however transcription always stops at the same polyadenylation site so that the overlapping mRNAs have a common 3' terminus. One of these mRNAs is slightly longer than the DNA sequence because of the polyadenylation at one end and a repeated region. This is the full length c-RNA that will be the template for the genome. The full length mRNA codes for the polymerase and core HBcAg and HBeAg proteins. The latter are very similar because they are translated in the same reading frame from two different start codons. Two smaller mRNAs (2.4 and 2.1 kilobases) which overlap code for the surface glycoproteins. There is also a small mRNA (700 bases) that codes for a protein that is a protein kinase and is a transactivator of transcription.
In the cytoplasm, the full-length (3,500 base) positive strand c-RNA is encapsidated by core proteins. Inside the core, the RNA is transcribed to minus strand DNA by the same DNA polymerase (reverse transcriptase) that completed the double-stranded DNA and, at the same time, the RNA is degraded by a ribonuclease H that is also part of the reverse transcriptase. Unlike the reverse transcriptase of the retroviruses, the HBV reverse transcription reaction does not require a primer. Rather, the polymerase itself acts as a primer and remains covalently attached to the 5’ end of the negative strand DNA. A host cell chaperone protein (heat shock protein 90) is also necessary. This chaperone associates with the reverse transcriptase allowing it to fold into an active conformation.
The virus now buds through the endoplasmic reticulum and/or Golgi body membranes of the host cell from which it acquires HBsAg. At this stage or later, the minus stand of DNA is partly transcribed into a plus strand. When the viral DNA polymerase is used to transcribe RNA to DNA, it is acting as a reverse transcriptase. This reverse transcriptase is similar to that found in retroviruses; in fact, HBV DNA polymerase and retroviral reverse transcriptase are so similar that they may have evolved from a common ancestor.
Virus particles that contain RNA or DNA at various stages of replication can be found in the bloodstream of an infected person, suggesting that nucleic acid replication is not tightly controlled with the passage out of the cell. Also, empty envelopes containing the envelope proteins embedded in a lipid bilayer are continuously being shed.[7]
Pathology
HBV causes acute and chronic hepatitis. Following the entry of HBV into the body, the virus targets hepatocytes (liver cells), most likely because its receptor is found largely on these cells. The incubation period of the virus is 60-90 days, and the rate at which symptoms appear depends on the initial dose of virus. The first sign of infection is enlarged hepatocytes and the characteristic appearance of HBsAg in infected cells, known as ground glass appearance. HBsAg is found associated with the endoplasmic reticulum and the cell nuclei have core particles containing HBcAg.
As with other hepatitis viruses, the symptoms are immune-mediated, resulting from inflammation and cell-mediated (cytotoxic T-cell) responses to HBsAg on the surface of hepatocytes, which also resolve the disease. Symptoms of hepatitis include jaundice, fatigue, abdominal pain, loss of appetite, nausea, vomiting and joint pain. The chance of becoming chronically infected depends upon age. If the cell-mediated immune response is weak, as with younger patients, symptoms are mild but the infection does not resolve and chronic hepatitis develops. About 90% of infected neonates and 50% of infected young children will become chronically infected. In contrast, only about 5% to 10% of immunocompetent adults infected with HBV develop chronic hepatitis B. In some individuals who become chronically infected, especially neonates and children, the acute infection will not be clinically apparent. These patients, who are still potentially infectious, have no symptoms and no abnormalities on laboratory testing. Nevertheless, some of these patients will have evidence of hepatitis on liver biopsy.[8]
Chronic infection
People with chronic HBV infection may have no evidence of liver disease or may have a spectrum of disease ranging from chronic hepatitis to cirrhosis or liver cancer. According to CDC, the laboratory criteria for diagnosis of hepatitis B chronic infection are IgM antibodies to hepatitis B core antigen (anti-HBc) negative and a positive result on one of the following tests: HBsAg, HBeAg, or HBV DNA. Or, HBsAg positive or HBV DNA positive or HBeAg positive two times at least 6 months apart (Any combination of these tests performed 6 months apart is acceptable). Approximately 25% of those who become chronically infected during childhood and 15% of those who become chronically infected after childhood die prematurely from cirrhosis or liver cancer, and the majority remain asymptomatic until onset of cirrhosis or end-stage liver disease.[9]
Chronic infection with HBV can be either "replicative" or "non-replicative." In non-replicative infection, the rate of viral replication in the liver is low and serum HBV DNA concentration is generally low and HBeAg is not detected. HBeAg is an alternatively processed protein of the pre-core gene that is only synthesized under conditions of high viral replication. In "replicative" infection, the patient usually has a relatively high serum concentration of viral DNA and detectable HBeAg. Patients with chronic hepatitis B and "replicative" infection defined by the presence of detectable HBeAg have a generally worse prognosis and a greater chance of developing cirrhosis and/or hepatocellular carcinoma than those without HBeAg. In rare strains of HBV with mutations in the pre-core gene, "replicative" infection can occur in the absence of detectable serum HBeAg.[10]
Some individuals with chronic hepatitis B will have clinically insignificant or minimal liver disease and never develop complications. Others will have clinically apparent chronic hepatitis. Some will go on to develop cirrhosis. Individuals with chronic hepatitis B are at increased risk for the development of hepatocellular carcinoma.
HBV is a major cause of hepatocellular carcinoma worldwide; in fact, HBV infection may be the cause of over 80% of primary hepatocellular carcinoma cases worldwide.[11] It is clear that individuals who are HBsAg positive are at a much higher risk of hepatocellular carcinoma than those who are negative. In Taiwan, where 15% of the population is carriers, HBsAg carriers have a risk of hepatocellular carcinoma that is 217 times that of a non-carrier. About half of deaths of HBsAg carriers are caused by liver cirrhosis or hepatocellular carcinoma compared to 2% of the general population.
In patients with chronic hepatitis, there is destruction of hepatocytes as a result of the immune response to the virus. This results in regeneration (by cell division) of liver cells that may ultimately cause the cancer. Although the virus does not integrate during the course of normal replication, parts of the HBV genome are found integrated into the DNA of hepatocellular carcinoma patients. This may result in the activation of a cellular proto-oncogene in much the same way as occurs in some retrovirus-caused cancers. Hepatocellular carcinoma takes many years to develop and this may reflect the rarity of integration in the absence of an integrase enzyme. The tumor that does develop is thus likely to be a clone of a single cell where this process has occurred. An HBV protein called protein X is known to activate the src kinase and this may also underlie HBV carcinogenesis. This protein may also interact with p53, one of the cell's tumor suppressor genes.[12]
Acute infection
An acute hepatitis B infection is defined by CDC as an infection with discrete onset of symptoms and either jaundice or elevated serum aminotransferase levels. The laboratory criteria for diagnosis of hepatitis B acute infection are IgM antibody to anti-HBc positive or HBsAg positive and IgM anti-HAV negative.[13]
Acute infection ranges from asymptomatic or mild disease to fulminant hepatic failure in about 2% of cases. The disease is more severe among elderly adults (age greater that 60 years) with symptoms typically lasting for several weeks but can persist for up to 6 months. The fatality rate among acute cases reported to CDC is 0.5% – 1%.
Many acutely infected individuals develop clinically apparent acute hepatitis with loss of appetite, nausea, vomiting, fever, abdominal pain and jaundice. In cases of fulminant hepatic failure from acute HBV infection, orthotopic liver transplantation can be life-saving.
Treatment
Clinical practice guidelines are available.[14]
There is no medication available for acute infection. The only type of treatment is supportive.
For chronic infection, several antiviral drugs (adefovir dipivoxil, interferon alfa-2b, pegylated interferon alfa-2a, lamivudine, entecavir, and telbivudine) are available. People with chronic HBV infection require medical evaluation and regular monitoring to determine whether the disease is progressing and to identify liver damage or hepatocellular carcinoma.
Prevention
The Centers for Disease Control and Prevention have issued recommendations for vaccination against hepatitis B among patients with diabetes.[15]
Current research
Chinese Herbal Medicine and Interferon in the Treatment of Chronic Hepatitis B: A Meta-Analysis of Randomized, Controlled Trials[16]
A study conducted by the University of California at Berkeley showed that a new Chinese-herbal treatment may be beneficial to treatment of chronic hepatitis B. The researchers compared changes in three markers of infection (HBsAg, HBeAg, and HBV) as a result of three types of treatments: Chinese herbal medicine alone, Chinese herbal treatment in conjugation with interferon alfa, and interferon alfa alone. The results showed that combination of Chinese herbal treatments with interferon alfa were 1.5 to 2 times more effective as interferon alfa in reducing the hepatitis B viral load to undetectable level for all three measure of infection. The herbal treatments used for this study included mixtures of bufotoxin (an extract from the skin of the toad bufo gargarizans) and kurorinone (an extract from the root of the pant sophorae flavescentis). Although the quality of this study was poor,because of unclear protocol, these data suggest that further trials of Chinese Herbal Medicine and interferon in chronic hepatitis B infection are justified.
Hepatitis B vaccine and the risk of CNS inflammatory demyelination in childhood[17]
The role of hepatitis B (HB) vaccine in the risk of CNS inflammatory demyelination is debated, with studies reporting conflicting findings. The objective of this study was to investigate whether vaccination against hepatitis B increases the risk of CNS inflammatory demyelination in children. To do this, the researchers conducted a population-based case-control study where the cases were 349 children with a first episode of acute CNS inflammatory demyelination in France (1994-2003). Each case was matched on age, sex, and geographic location to up to 12 controls, randomly selected from the general population. Information on vaccinations was confirmed by a copy of the vaccination certificate. The odds ratios of CNS inflammatory demyelination associated with HB vaccination were estimated using conditional logistic regression. The rates of HB vaccination in the 3 year period prior the index date were 24.4% for the 349 cases and 27.3% for their 2,941 matched controls. The results showed that there was no connection between HB vaccination within this 3 year period (nor after it) and the rate of CSN inflammatory demyelination. However, a specific HB vaccine - Engerix B, seemed to increase this risk in the longer term.
This study demonstrated that Hepatitis B vaccination does not generally increase the risk of CNS inflammatory demyelination in childhood. This study is important for two major reasons. First, it will alleviate some parents' fears from vaccinating their children for hepatitis B, a life-threatening disease. Second, it raises a concern about a specific brand of a hepatitis B vaccine that should be further tested and evaluated for safety.
References
- ↑ http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm#overview
- ↑ http://web.uct.ac.za/depts/mmi/stannard/hepb.html
- ↑ http://www.ncbi.nlm.nih.gov/sites/entrez?Db=genomeprj&Cmd=Retrieve&list_uids=15428
- ↑ http://www.ncbi.nlm.nih.gov/sites/entrez?Db=genomeprj&Cmd=Retrieve&list_uids=15428
- ↑ http://www.cumc.columbia.edu/dept/gi/hepB.html
- ↑ http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm#treatment
- ↑ http://pathmicro.med.sc.edu/virol/hepatitis-virus.htm
- ↑ http://pathmicro.med.sc.edu/virol/hepatitis-disease2.htm
- ↑ http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm#overview
- ↑ http://www.cumc.columbia.edu/dept/gi/hepB.html
- ↑ http://pathmicro.med.sc.edu/virol/hepatitis-disease2.htm
- ↑ http://pathmicro.med.sc.edu/virol/hepatitis-virus.htm
- ↑ http://www.cdc.gov/ncphi/disss/nndss/casedef/hepatitisb2000.htm
- ↑ Lok AS, McMahon BJ (2009). "Chronic hepatitis B: update 2009.". Hepatology 50 (3): 661-2. DOI:10.1002/hep.23190. PMID 19714720. Research Blogging.
- ↑ Centers for Disease Control and Prevention (CDC) (2011). "Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP).". MMWR Morb Mortal Wkly Rep 60 (50): 1709-11. PMID 22189894. [e]
- ↑ McCulloch, M (2002) Chinese Herbal Medicine and Interferon in the Treatment of Chronic Hepatitis B: A Meta-Analysis of Randomized, Controlled Trials. American Journal of Public Health 92: 1619-1628.
- ↑ Mikaeloff, Y (2009) Hepatitis B vaccine and the risk of CNS inflammatory demyelination in childhood. NEUROLOGY 72: 873-880.