Fluid catalytic cracking: Difference between revisions
imported>Milton Beychok m (Minor copy edits) |
imported>Milton Beychok m (→Catalysts: Minor addition) |
||
| Line 118: | Line 118: | ||
* Low coke production | * Low coke production | ||
A modern FCC catalyst has four major components: crystalline [[zeolite]], matrix, binder and filler. Zeolite is the primary active component and can range from about 15 to 50 weight percent of the catalyst. The zeolite is comprised of [[silica]] and [[alumina]] tetrahedra with each tetrahedron having either an [[aluminum]] or a [[silicon]] atom at the center and four [[oxygen]] atoms at the corners. It is a [[molecular sieve]] with a distinctive lattice structure that allows only a certain size range of hydrocarbon molecules to enter the lattice. In general, the zeolite does not allow molecules large that 8 to 10 nm (i.e., [[angstrom (unit)|angstroms]]) to enter the lattice.<ref name=Kogel/><ref name=Yang/> | A modern FCC catalyst has four major components: crystalline [[zeolite]], matrix, binder and filler. Zeolite is the primary active component and can range from about 15 to 50 weight percent of the catalyst. The zeolite used in FCC catalysts is referred to ''faujasite'' or as ''Type Y'' and is comprised of [[silica]] and [[alumina]] tetrahedra with each tetrahedron having either an [[aluminum]] or a [[silicon]] atom at the center and four [[oxygen]] atoms at the corners. It is a [[molecular sieve]] with a distinctive lattice structure that allows only a certain size range of hydrocarbon molecules to enter the lattice. In general, the zeolite does not allow molecules large that 8 to 10 nm (i.e., [[angstrom (unit)|angstroms]]) to enter the lattice.<ref name=Kogel/><ref name=Yang/> | ||
The catalytic sites in the zeolite are strong acids (equivalent to 90% [[sulfuric acid]]) and provide most of the catalyst activity. The acidic sites are provided by the alumina tetrahedra. The aluminum atom at the center of each alumina tetrahedra is at a +3 [[oxidation state ]] surrounded by four oxygen atoms at the corners which are shared by the neighboring tetrahedra. Thus, the net charge of the alumina tetrahedra is -1 which is balanced by a [[sodium]] [[ion]] during the production of the catalyst. The sodium ion is later replaced by an [[ammonium]] ion which is vaporized when the catalyst is subsequently dried, resulting in the formation of [[Lewis acid|Lewis]] and [[Brønsted acid|Brønsted]] acidic sites. In some FCC catalysts, the Brønsted sites may be later replaced by [[rare earth]] metals such as [[cerium]] and [[lanthanum]] to provide alternative activity and stability levels.<ref name=Kogel/><ref name=Yang/> | The catalytic sites in the zeolite are strong acids (equivalent to 90% [[sulfuric acid]]) and provide most of the catalyst activity. The acidic sites are provided by the alumina tetrahedra. The aluminum atom at the center of each alumina tetrahedra is at a +3 [[oxidation state ]] surrounded by four oxygen atoms at the corners which are shared by the neighboring tetrahedra. Thus, the net charge of the alumina tetrahedra is -1 which is balanced by a [[sodium]] [[ion]] during the production of the catalyst. The sodium ion is later replaced by an [[ammonium]] ion which is vaporized when the catalyst is subsequently dried, resulting in the formation of [[Lewis acid|Lewis]] and [[Brønsted acid|Brønsted]] acidic sites. In some FCC catalysts, the Brønsted sites may be later replaced by [[rare earth]] metals such as [[cerium]] and [[lanthanum]] to provide alternative activity and stability levels.<ref name=Kogel/><ref name=Yang/> | ||
Revision as of 01:01, 8 May 2008
The fluid catalytic cracker (FCC) is the most important conversion process used in petroleum refineries. It is widely used to convert the high-boiling hydrocarbon fractions of petroleum crude oils to more valuable gasoline, olefinic gases and other products.[1][2][3] Catalytic cracking of petroleum hydrocarbons was originally done by thermal cracking which has been almost completely replaced by catalytic cracking because it produces more gasoline with a higher octane rating. It also produces byproduct gases that are more olefinic, and hence more valuable, than those produced by thermal cracking.
The feedstock to an FCC is usually that portion of the crude oil that has an initial boiling point of 340 °C or higher at atmospheric pressure and an average molecular weight ranging from about 200 to 600 or higher. The FCC process vaporizes and breaks the long-chain molecules of the high-boiling hydrocarbon liquids into much shorter molecules by contacting the feedstock, at high temperature and moderate pressure, with a fluidized powdered catalyst.
In effect, refineries use fluid catalytic cracking to correct the imbalance between the market demand for gasoline and the excess of heavy, high boiling range products resulting from the distillation of crude oil.
As of 2006, FCC units were in operation at 400 petroleum refineries worldwide and about one-third of the crude oil refined in those refineries is processed in an FCC to produce high-octane gasoline and fuel oils.[2][4] During 2007, the FCC units in the United States processed a total of 834,300,000 litres (5,300,000 barrels per day of feedstock[5] and FCC units worldwide processed about twice that amount.
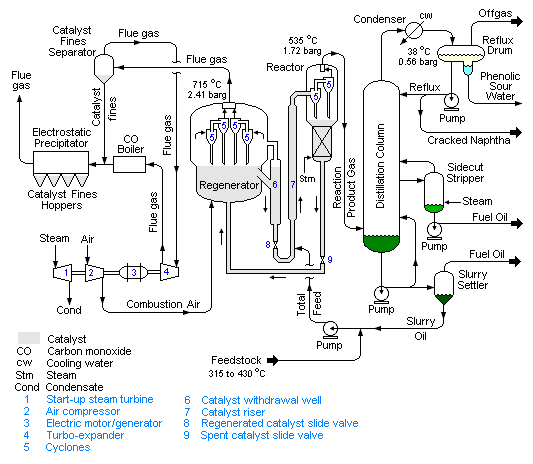
Flow diagram and process description
The modern FCC units are all continuous processes which operate 24 hours a day for as much as 2 to 3 years between shutdowns for routine maintenance.
There are a number of different proprietary designs that have been developed for modern FCC units. Each design is available under a license that must be purchased from the design developer by any petroleum refining company desiring to construct and operate an FCC of a given design.
Basically, there are two different configurations for an FCC unit: the "stacked" type where the reactor and the catalyst regenerator are contained in a single vessel with the reactor above the catalyst regenerator and the "side-by-side" type where the reactor and catalyst regenerator are in two separate vessels. These are the major FCC designers and licensors:[1][3][4][6]
Side-by-side configuration:
- ABB Lummus Global
- Exxon Research and Engineering (ERE)
- Shell Global Solutions International
- Stone & Webster Engineering Corporation (SWECO) / Institut Francais Petrole (IFP)
- Universal Oil Products (UOP)
Stacked configuration:
- Kellogg Brown & Root (KBR)
Each of the proprietary design licensors claims to have unique features and advantages. A complete discussion of the relative advantages of each of the processes is well beyond the scope of this article. Suffice it to say that all of the licensors have designed and constructed FCC units that have operated quite satisfactorily.
Reactor and Regenerator
The schematic flow diagram of a typical modern FCC unit in Figure 1 below is based upon one of the above configurations. The preheated high-boiling petroleum feedstock (at about 315 to 430 °C) consisting of long-chain hydrocarbon molecules is combined with recycle slurry oil from the bottom of the distillation column and injected into the catalyst riser where it is vaporized and cracked into smaller molecules of vapor by contact and mixing with the very hot powdered catalyst from the regenerator. All of the cracking reactions take place in the catalyst riser. The hydrocarbon vapors "fluidize" the powdered catalyst and the mixture of hydrocarbon vapors and catalyst flows upward to enter the reactor at a temperature of about 535 °C and a pressure of about 1.72 barg.
The reactor is in fact merely a vessel in which the cracked product vapors are: (a) separated from the so-called spent catalyst by flowing through a set of two-stage cyclones within the reactor and (b) the spent catalyst flows downward through a steam stripping section to remove any hydrocarbon vapors before the spent catalyst returns to the catalyst regenerator. The flow of spent catalyst to the regenerator is regulated by a slide valve in the spent catalyst line.
Since the cracking reactions produce some carbonaceous material (referred to as coke) that deposits on the catalyst and very quickly reduces the catalyst reactivity, the catalyst is regenerated by burning off the deposited coke with air blown into the regenerator. The regenerator operates at a temperature of about 715 °C and a pressure of about 2.41 barg. The combustion of the coke is exothermic and it produces a large amount of heat that is partially absorbed by the regenerated catalyst and provides the heat required for the vaporization of the feedstock and the endothermic cracking reactions that take place in the catalyst riser. For that reason, FCC units are often referred to as being heat balanced.
The hot catalyst (at about 715 °C) leaving the regenerator flows into a catalyst withdrawal well where any entrained combustion flue gases are allowed to escape and flow back into the upper part to the regenerator. The flow of regenerated catalyst to the feedstock injection point below the catalyst riser is regulated by a slide valve in the regenerated catalyst line. The hot flue gas exits the regenerator after passing through multiple sets of two-stage cylones that remove entrained catalyst from the flue gas,
The amount of catalyst circulating between the regenerator and the reactor amounts to about 5 kg per kg of feedstock which is equivalent to about 4.66 kg per litre of feedstock.[1][7] Thus, an FCC unit processing 12,000,000 litres/day (75,000 barrels/day) will circulate about 55,900 metric tons per day of catalyst.
Distillation column
The reaction product vapors (at 535 °C and a pressure of 1.72 barg) flow from the top of the reactor to the bottom section of the distillation column (commonly referred to as the main fractionator) where they are distilled into the FCC end products of cracked naphtha, fuel oil and offgas. After further processing for removal of sulfur compounds, the cracked naphtha becomes a high-octane component of the refinery's blended gasolines.
The main fractionator offgas is sent to what is called a gas recovery unit where it is separated into butanes and butylenes, propane and propylene, and lower molecular weight gases (hydrogen, methane, ethylene and ethane). Some FCC gas recovery units may also separate out some of the ethane and ethylene.
Although the schematic flow diagram above depicts the main fractionator as having only one sidecut stripper and one fuel oil product, many FCC main fractionators have two sidecut strippers and produce a light fuel oil and a heavy fuel oil. Likewise, many FCC main fractionators produce a light cracked naphtha and a heavy cracked naphtha. The terminology light and heavy in this context refers to the product boiling ranges, with light products having a lower boiling range than heavy products.
The bottom product oil from the main fractionator contains residual catalyst particles which were not completely removed by the cyclones in the top of the reactor. For that reason, the bottom product oil is referred to as a slurry oil. Part of that slurry oil is recycled back into the main fractionator above the entry point of the hot reaction product vapors so as to cool and partially condense the reaction product vapors as they enter the main fractionator. The remainder of the slurry oil is pumped through a slurry settler. The bottom oil from the slurry settler contains most of the slurry oil catalyst particles and is recycled back into the catalyst riser by combining it with the FCC feedstock oil. The so-called clarified slurry oil is withdrawn from the top of slurry settler for use elsewhere in the refinery or as a heavy fuel oil blending component.
Regenerator flue gas
Depending on the choice of FCC design, the combustion in the regenerator of the coke on the spent catalyst may or may not be complete combustion to carbon dioxide (CO2). The combustion air flow is controlled so as to provide the desired ratio of carbon monoxide (CO) to carbon dioxide for each specific FCC design.[1] [4]
In the design shown in Figure 1, the coke has only been partially combusted to CO2. The combustion flue gas (containing CO and CO2) at 715 °C and at a pressure of 2.41 barg is routed through a secondary catalyst separator containing swirl tubes designed to remove 70 to 90 percent of the particulates in the flue gas leaving the regenerator.[8] This is required to prevent erosion damage to the blades in the turbo-expander that the flue gas is next routed through.
The expansion of flue gas through a turbo-expander provides sufficient power to drive the regenerator's combustion air compressor. The electrical motor-generator can consume or produce electrical power. If the expansion of the flue gas does not provide enough power to drive the air compressor, the electric motor/generator provides the needed additional power. If the flue gas expansion provides more power than needed to drive the air compressor, than the electric motor/generator converts the excess power into electric power and exports it to the refinery's electrical system.[3]
The expanded flue gas is then routed through a steam-generating boiler (referred to as a CO boiler) where the carbon monoxide in the flue gas is burned as fuel to provide steam for use in the refinery as well as to comply with any applicable environmental regulatory limits on carbon monoxide emissions.[3]
The flue gas is finally processed through an electrostatic precipitator (ESP) to remove residual particulate matter to comply with any applicable environmental regulations regarding particulate emissions. The ESP removes particulates in the size range of 2 to 20 microns from the flue gas.[3]
The steam turbine in the flue gas processing system (shown in the above diagram) is used to drive the regenerator's combustion air compressor during start-ups of the FCC unit until there is sufficient combustion flue gas to take over that task.
Chemistry
Before delving into the chemistry involved in catalytic cracking, it will be helpful to briefly discuss the composition of petroleum crude oil.
Petroleum crude oil consists primarily of a mixture of hydrocarbons with small amounts of other organic compounds containing sulfur, nitrogen and oxygen. The crude oil also contains small amounts of metals such as copper, iron, nickel and vanadium.[2]
| Carbon | 83-87% |
| Hydrogen | 10-14% |
| Nitrogen | 0.1-2% |
| Oxygen | 0.1-1.5% |
| Sulfur | 0.5-6% |
| Metals | < 0.1% |
The elemental composition ranges of crude oil are summarized in Table 1 and the hydrocarbons in the crude oil can be classified into three types:[1][2]
- Paraffins or alkanes: saturated straight-chain or branched hydrocarbons, without any ring structures
- Naphthenes or cycloalkanes: saturated hydrocarbons having one or more ring structures with one or more side-chain paraffins
- Aromatics: hydrocarbons having one or more unsaturated ring structures such as benzene or unsaturated polycyclic ring structures such as naphthalene or phenanthrene, any of which may also have one or more side-chain paraffins.
Olefins or alkenes, which are unsaturated straight-chain or branched hydrocarbons, do not occur naturally in crude oil.
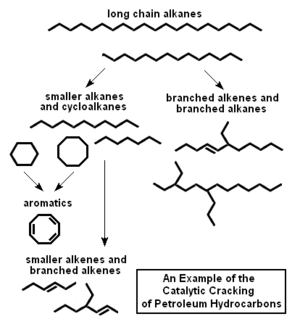
In plain language, the fluid catalytic cracking process breaks large hydrocarbon molecules into smaller molecules by contacting them with powdered catalyst at a high temperature and moderate pressure which first vaporizes the hydrocarbons and then breaks them. The cracking reactions occur in the vapor phase and start immediately when the feedstock is vaporized in the catalyst riser.
Figure 2 is a very simplified schematic diagram that exemplifies how the process breaks high boiling, straight-chain alkane (paraffin) hydrocarbons into smaller straight-chain alkanes as well as branched-chain alkanes, branched alkenes (olefins) and cycloalkanes (naphthenes).[9] The breaking of the large hydrocarbon molecules into smaller molecules is more technically referred to by organic chemists as scission of the carbon-to-carbon bonds.
As depicted in Figure 2, some of the smaller alkanes are then broken and converted into even smaller alkenes and branched alkenes such as the gases ethylene, propylene, butylenes and isobutylenes. Those olefinic gases are valuable for use as petrochemical feedstocks. The propylene, butylene and isobutylene are also valuable feedstocks for certain petroleum refining processes that convert them into high-octane gasoline blending components.
As also depicted in Figure 2, the cycloalkanes (naphthenes) formed by the initial breakup of the large molecules are further converted to aromatics such as benzene, toluene and xylenes which boil in the gasoline boiling range and have much higher octane ratings than alkanes.
By no means does Figure 2 include all the chemistry of the primary and secondary reactions taking place in the fluid catalytic process. There are a great many other reactions involved. However, a full discussion of the highly technical details of the various catalytic cracking reactions is beyond the scope of this article and can be found in the technical literature.[1][2][3][4]
Catalysts
Modern FCC catalysts are fine powders with a bulk density of 0.80 to 0.96 g/cc and having a particle size distribution ranging from 10 to 150 μm and an average particle size of 60 to 100 μm.[10][11] The design and operation of an FCC unit is largely dependent upon the chemical and physical properties of the catalyst. The desirable properties of an FCC catalyst are:
- Good stability to high temperature and to steam
- High activity
- Large pore sizes
- Good resistance to attrition
- Low coke production
A modern FCC catalyst has four major components: crystalline zeolite, matrix, binder and filler. Zeolite is the primary active component and can range from about 15 to 50 weight percent of the catalyst. The zeolite used in FCC catalysts is referred to faujasite or as Type Y and is comprised of silica and alumina tetrahedra with each tetrahedron having either an aluminum or a silicon atom at the center and four oxygen atoms at the corners. It is a molecular sieve with a distinctive lattice structure that allows only a certain size range of hydrocarbon molecules to enter the lattice. In general, the zeolite does not allow molecules large that 8 to 10 nm (i.e., angstroms) to enter the lattice.[10][11]
The catalytic sites in the zeolite are strong acids (equivalent to 90% sulfuric acid) and provide most of the catalyst activity. The acidic sites are provided by the alumina tetrahedra. The aluminum atom at the center of each alumina tetrahedra is at a +3 oxidation state surrounded by four oxygen atoms at the corners which are shared by the neighboring tetrahedra. Thus, the net charge of the alumina tetrahedra is -1 which is balanced by a sodium ion during the production of the catalyst. The sodium ion is later replaced by an ammonium ion which is vaporized when the catalyst is subsequently dried, resulting in the formation of Lewis and Brønsted acidic sites. In some FCC catalysts, the Brønsted sites may be later replaced by rare earth metals such as cerium and lanthanum to provide alternative activity and stability levels.[10][11]
The matrix component of an FCC catalyst contains amorphous alumina which also provides catalytic activity sites and in larger pores that allows entry for larger molecules than does the zeolite. That enables the cracking of higher-boiling, larger feedstock molecules than are cracked by the zeolite.
The binder and filler components provide the physical strength and integrity of the catalyst. The binder is usually silica sol and the filler is usually a clay (kaolin).
Nickel, vanadium, iron, copper and other metal contaminants, present in FCC feedstocks in the parts per million range, all have detrimental effects on the catalyst activity and performance. Nickel and vanadium are particularly troublesome. There are a number of methods for mitigating the effects of the contaminant metals:[12][13]
- Avoid feedstocks with high metals content: This seriously hampers a refinery's flexibility to process various crude oils or purchased FCC feedstocks.
- Feedstock feed pretreatment: Hydrodesulfurization of the FCC feedstock removes some of the metals and also reduces the sulfur content of the FCC products. However, this is quite a costly option.
- Increasing fresh catalyst addition: All FCC units withdraw some of the circulating equilibrium catalyst as spent catalyst and replaces it with fresh catalyst in order to maintain a desired level of activity. Increasing the rate of such exchange lowers the level of metals in the circulating equilibrium catalyst, but this is also quite a costly option.
- Demetallization: The commercial proprietary Demet Process removes nickel and vanadium from the withdrawn spent catalyst. The nickel and vanadium are converted to chlorides which are then washed out of the catalyst. After drying, the demetallized catalyst is recycled into the circulating catalyst. Removals of about 95 percent nickel removal and 67 to 85 percent vanadium have been reported. Despite that, the use of the Demet process has not become widespread, perhaps because of the high capital expenditure required.
- Metals passivation: Certain materials can be used as additives which can be impregnated into the catalyst or added to the FCC feedstock in the form of metal-organic compounds. Such materials react with the metal contaminants and passivate the contaminants by forming less harmful compounds that remain on the catalyst. For example, antimony and bismuth are effective in passivating nickel and tin is effective in passivating vanadium. A number of proprietary passivation processes are available and fairly widely used.
The major suppliers of FCC catalysts worldwide include Albemarle Corporation, W.R. Grace Company and BASF Catalysts (formerly Engelhard).
History
(This section is being worked on. Will be ready in 2-3 days.)
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 James H. Gary and Glenn E. Handwerk (2001). Petroleum Refining: Technology and Economics, 4th Edition. CRC Press. ISBN 0-8247-0482-7.
- ↑ 2.0 2.1 2.2 2.3 2.4 James. G. Speight (2006). The Chemistry and Technology of Petroleum, 4th Edition. CRC Press. ISBN 0-8493-9067-2.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Reza Sadeghbeigi (2000). Fluid Catalytic Cracking Handbook, 2nd Edition. Gulf Publishing. ISBN 0-88415-289-8.
- ↑ 4.0 4.1 4.2 4.3 David S.J. Jones and Peter P. Pujado (Editors) (2006). Handbook of Petroleum Processing, First Edition. Springer. ISBN 1-4020-2819-9.
- ↑ U.S. Downstream Processing of Fresh Feed Input by Catalytic Cracking Units (Energy Information Administration, U.S. Dept. of Energy)
- ↑ Editorial Staff (November 2002). "Refining Processes 2002". Hydrocarbon Processing: 108-112. ISSN 0887-0284.
- ↑ Fluid Catalytic Cracking
- ↑ Alex C. Hoffnab and Lewis E. Stein (2002). Gas Cyclones and Swirl Tubes:Principles , Design and Operation, 1st Edition. Springer. ISBN 3-540-43326-0.
- ↑ Fluid catalytic cracking from the website funded by the Institute of Applied Catalysis (IAC) and the Chemical Industry Education Centre (CIEC)
- ↑ 10.0 10.1 10.2 Jessica Elzea Kogel, Nikhil C. Trivedi, James M. Barber and Stanley T. Krukowsk (Editors) (2006). Industrial Minerals & Rocks: Commodities, Markets and Uses, Seventh Edition. Society of Mining, Metallurgy and Exploration. ISBN 0-87335-233-5.
- ↑ 11.0 11.1 11.2 Wen-Ching Yang (2003). Handbook of Fluidization and Fluid Particle Systems. CRC Press. ISBN 0-8247-0259-X.
- ↑ Passivate Vanadium on FCC Catalysts for Improved Refinery Profitability (1997 Annual National Petrochemical and Refiners Association (NPRA) Meeting)
- ↑ Julius Scherzer (1990). Octane-enhancing Zeolitic FCC Catalysts: Scientific ans Technical Aspects. CRC Press. ISBN 0-8247-8399-9.