Ketoconazole: Difference between revisions
imported>David E. Volk mNo edit summary |
imported>David E. Volk (→Synonyms: Brand names too) |
||
| Line 29: | Line 29: | ||
* orifungal M | * orifungal M | ||
* panfungol | * panfungol | ||
== Brand names == | |||
* Extina® | |||
* Fungarest® | |||
* Fungoral® | |||
* Ketocanazole® | |||
* Ketoconazol® | |||
* Ketoconazol® | |||
* Ketoconazole® | |||
* Ketoconazolum® | |||
* Ketoderm® | |||
* Ketoisdin® | |||
* Ketozole® | |||
* Nizoral® | |||
* Nizoral Cream® | |||
* Nizoral Shampoo® | |||
* Nizoral a-D® | |||
* Nizoral a-D Shampoo® | |||
* Orifungal® | |||
* Orifungal M® | |||
* Panfungol® | |||
== External links == | == External links == | ||
Revision as of 15:58, 3 March 2008
Ketoconazole is one of the azole-based antifungal drugs used to treat fungal infections. Its use has greatly diminished because of the introduction of better treatment alternatives, including the triazoles fluconazole and itraconazole. It is based on imidazole as are clotrimazole, fluconazole, itraconazole and miconazole. It is a second-line of defense systemic drug use to treat candidiasis, candiduria, oral thrush, mucocutaneious candidiasis, blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis and paracocccidioidomycosis.
Mechanism of action
Azole-based antifungal agents, such as ketoconazole, work by inhibiting the enzyme cytochrome P450 14--demethylase (P45014DM), which is part of the sterol biosynthesis pathway that converts lanosterol to ergosterol[1]. Because ketoconazole has less affinity towards fungal cell membranes than the newer triazole antifungal agents like fluconazole and itraconazole, it is more likely to bind with mammalian cell membranes and induce toxicity[2]. .
Side effects
Becuase ketoconazole disrupts part of the sterol biosynthesis pathway, it may decrease levels of the steroids testosterone and cortisone causing gynecomastia and oligospermia in males and irregular menstration in women. It may also cause anorexia, nausea and vomiting, increased levels of transaminase and liver toxicity.
Chemistry
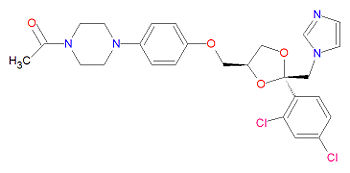
The IUPAC chemical name for ketoconazole is 1-[4-[4-[[2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]ethanone. It has chemical formula C26H28Cl2N4O4 and registered under CAS Number 79156-75-5.
Synonyms
- (+-)-cis-1-acetyl-4-(p-((2-(2,4-dichlorophenyl)-2-(imidazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)piperazine,
- 79156-75-5
- CPD-4503
- fungarest
- fungoral,
- ketoconazol,
- ketoconazole
- ketoderm
- ketoisdin,
- KW 1414
- NCI60_002728
- nizoral
- NSC317629
- orifungal M
- panfungol
Brand names
- Extina®
- Fungarest®
- Fungoral®
- Ketocanazole®
- Ketoconazol®
- Ketoconazol®
- Ketoconazole®
- Ketoconazolum®
- Ketoderm®
- Ketoisdin®
- Ketozole®
- Nizoral®
- Nizoral Cream®
- Nizoral Shampoo®
- Nizoral a-D®
- Nizoral a-D Shampoo®
- Orifungal®
- Orifungal M®
- Panfungol®
External links
- Doctorfungus [1]