User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok |
imported>Milton Beychok No edit summary |
||
| Line 21: | Line 21: | ||
*The activation energy of the catalyzed reaction is significantly smaller than that of the uncatalyzed reaction. Hence, the rate of the catalyzed reaction is much faster. | *The activation energy of the catalyzed reaction is significantly smaller than that of the uncatalyzed reaction. Hence, the rate of the catalyzed reaction is much faster. | ||
*Since the overall change in Gibbs free energy is the same for the catalyzed reaction as for the uncatalyzed reaction, the [[reaction equilibrium constant]] is not affected by the catalyst. As noted above, the catalyst does not change the chemical thermodynamics of the reaction. Thus, if a reaction is thermodynamically unfavorable, the catalyst cannot change that situation. | *Since the overall change in Gibbs free energy is the same for the catalyzed reaction as for the uncatalyzed reaction, the [[reaction equilibrium constant]] is not affected by the catalyst. As noted above, the catalyst does not change the chemical thermodynamics of the reaction. Thus, if a reaction is thermodynamically unfavorable, the catalyst cannot change that situation. | ||
==Types of catalysts== | |||
==Inhibitors, promoters and poisons== | |||
==Examples of catalyzed reactions== | |||
==History== | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Revision as of 22:20, 6 April 2010
In chemistry, Catalysis is a process that uses a substance to accelerate the rate of a chemical reaction through an uninterrupted and repeated cycle of elementary steps until the last step regenerates the catalyst in its original form. The substance that does this is known as a catalyst. It is usually present in relatively small amounts and none of it is consumed in the process.[1]
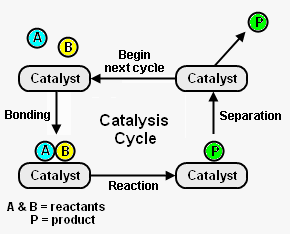
Figure 1 depicts the steps in a typical catalysis cycle. As depicted, the reactant molecules A and B are reacted to yield product P. The catalysis cycle starts with the bonding of reactant molecules A and B to the catalyst. A and B then react to yield product P which is also bound to the catalyst. In the last step, the catalyst is regenerated by product P separating from the catalyst. The regenerated catalyst then begins cycle again by bonding with two more reactant molecules. [2]
Many substances can act as catalysts, including: metals, chemical compounds (e.g., metal oxides, sulfides, nitrides), organometallic complexes, and enzymes. Although a catalyst may be a gas, liquid or solid, most catalysts used in industrial chemical reactions are in the form of porous pellets. Since not all parts of a solid catalyst participate in the catalysis cycle, those parts that do participate are referred to as active sites. A single porous pellet may have 1018 active catalytic sites.[1]
The catalysis mechanism
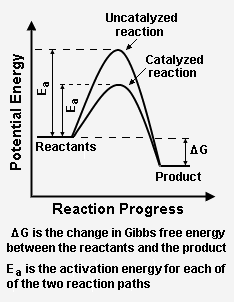
Figure 2: The effect of a catalyst on a hypothetical chemical reaction. The catalyst provides a reaction path with a lower activation energy than the uncatalyzed reaction path.[3]
In chemistry, activation energy[4] is the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a designated chemical reaction. It is denoted by Ea in units of kilojoules per mole (kJ/mol). It may be thought of as the energy barrier that must be overcome to start a chemical reaction.
For a chemical reaction to proceed at a reasonable rate, there should exist an appreciable number of reactant species (molecules, atoms, ions, etc.) with energy equal to or greater than the activation energy of the reaction.[3] A catalyst does not lower the activation energy for a reaction, instead it provides an alternative path for the reaction that has a lower activation energy. The catalyst changes the chemical kinetics of a reaction but not the chemical thermodynamics.
Figure 2 depicts how a catalyzed reaction follows a lower activation energy path than the higher activation energy path followed by the same reaction when it is not catalyzed. Overall, both the catalyzed path and the uncatalyzed path have the same change in Gibbs free energy between the reactants and the reaction product.
The energy diagram in Figure 2 illustrates several important points:
- The presence of the catalyst provides an an alternative point, which is definitely more complex (see Figure 1), but energetically much more favorable.
- The activation energy of the catalyzed reaction is significantly smaller than that of the uncatalyzed reaction. Hence, the rate of the catalyzed reaction is much faster.
- Since the overall change in Gibbs free energy is the same for the catalyzed reaction as for the uncatalyzed reaction, the reaction equilibrium constant is not affected by the catalyst. As noted above, the catalyst does not change the chemical thermodynamics of the reaction. Thus, if a reaction is thermodynamically unfavorable, the catalyst cannot change that situation.
Types of catalysts
Inhibitors, promoters and poisons
Examples of catalyzed reactions
History
References
- ↑ 1.0 1.1 Commission on Physical Sciences, Mathematics, and Applications (CPSMA), National Academies (1992). Catalysis Looks to the Future. National Academies Press. ISBN 0-309-04584-3. Available online at Executive Summary
- ↑ I. Chorkendorff and J. W. Niemantsverdriet (2007). Concepts of Modern Catalysts and Kinetics, 2nd Edition. Wiley-VCH. ISBN 3-527-31672-8.
- ↑ 3.0 3.1 The Effect of Catalysts on Reaction Rates Website provided by Jim Clarke, retired Head of Chemistry and then Head of Science at Truro School in Cornwall, United Kingdom.
- ↑ A term introduced in 1889 by the Swedish scientist Svante Arrhenius