User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok |
||
| Line 27: | Line 27: | ||
==Laboratory-scale vacuum distillation== | ==Laboratory-scale vacuum distillation== | ||
[[Image:Vacuum distillation of DMSO at 70C.jpg|right|thumb| | [[Image:Vacuum distillation of DMSO at 70C.jpg|right|thumb|165px|{{#ifexist:Template:Vacuum distillation of DMSO at 70C.jpg/credit|{{Vacuum distillation of DMSO at 70C.jpg/credit}}<br/>|}} [[Dimethyl sulfoxide]] boils at 189 °C at atmospheric pressure. Under vacuum, it distills at 70 °C.]] | ||
Laboratory-scale vacuum distillation, sometimes referred to as ''low temperature distillation'', is used when the liquids to be distilled have high [[atmospheric boiling points]] or undergo a chemical change at temperatures near their atmospheric boiling points.<ref>[http://www.epa.gov/esd/chemistry/vacuum/default.htm Vacuum Distillation: New Method for Analyzing Organic Chemicals in a Wide Array of Samples] ([[United States Environmental Protection Agency]])</ref><ref>[http://www.newton.dep.anl.gov/askasci/chem00/chem00635.htm What is vacuum distillation?] ([[Argonne National Laboratory]]'s NEWTON Ask-A-Scientist)</ref> | Laboratory-scale vacuum distillation, sometimes referred to as ''low temperature distillation'', is used when the liquids to be distilled have high [[atmospheric boiling points]] or undergo a chemical change at temperatures near their atmospheric boiling points.<ref>[http://www.epa.gov/esd/chemistry/vacuum/default.htm Vacuum Distillation: New Method for Analyzing Organic Chemicals in a Wide Array of Samples] ([[United States Environmental Protection Agency]])</ref><ref>[http://www.newton.dep.anl.gov/askasci/chem00/chem00635.htm What is vacuum distillation?] ([[Argonne National Laboratory]]'s NEWTON Ask-A-Scientist)</ref> | ||
| Line 38: | Line 38: | ||
[[Rotary evaporation]] is a type of vacuum distillation apparatus | [[Rotary evaporation]] is a type of vacuum distillation apparatus | ||
used to remove bulk [[solvent]]s from the liquid being distilled. Typically the vacuum in such systems is generated by a water [[aspirator]] or a [[membrane pump]]. | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Revision as of 23:02, 9 February 2008
Vacuum distillation is distillation of liquids performed at a pressure lower than atmospheric pressure to take advantage of the fact that reducing the pressure lowers the boiling point of liquids. This permits the distillation of liquids that are temperature sensitive and avoids any degradation of such liquids.
Vacuum distillation in petroleum refining
Petroleum crude oil is a complex mixture of hundreds of different hydrocarbon compounds generally having from 3 to 60 carbon atoms per molecule, although there may be small amounts of hydrocarbons outside that range.[1][2][3] In refining the crude oil, it is important not to subject the high molecular weight components to temperatures above 370 to 380 °C because they will undergo thermal cracking and form petroleum coke at temperatures above that. Formation of coke would result in plugging the tubes in the furnace that heats the feed stream to a column distilling either the whole crude oil or only the higher molecular weight components of crude oil. Plugging would also occur in the piping from the furnace to the distillation column as well as in the column itself.
The refining of crude oil begins with distilling the incoming crude oil in a so-called atmospheric distillation column operating at pressures slightly above atmospheric pressure.[1][2][4] The constraint imposed by limiting the column inlet
temperature to no more than 370 to 380 °C yields a residual oil from the bottom of the atmospheric distillation column consisting entirely of hydrocarbons that boil above 370 to 380 °C.
To further distill the residual oil from the atmospheric distillation column, the distillation must be performed at absolute pressures as low as 10 to 40 mmHg (also referred to as Torr) so as to limit the operating temperature to less than 370 to 380 °C.
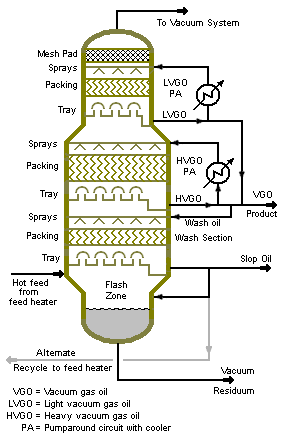
Figure 1 is a photograph of a large vacuum distillation column in a petroleum refinery and Figure 2 is a process diagram of a petroleum refinery vacuum distillation column that depicts the internals of the column.
The 10 to 40 mmHg absolute pressure in a vacuum distillation column increases the volume of vapor formed per volume of liquid distilled. The result is that such columns have very large diameters.[5]
Distillation columns such those in Figures 1 and 2, may have diameters of 15 meters or more, heights ranging up to about 50 meters, and feed rates ranging up to about 25,400 cubic meters per day (160,000 barrels per day).
The vacuum distillation column internals must provide good vapor-liquid contacting while, at the same time, maintaining a very low pressure increase from the top of the column top to the bottom. Therefore, the vacuum column uses distillation trays only where withdrawing products from the side of the column (referred to as side draws). Most of the column uses packing material for the vapor-liquid contacting because such packing has a lower pressure drop than distillation trays. This packing material can be either structured sheet metal or randomly dumped packing such as Raschig rings.
The absolute pressure of 10 to 40 mmHg in the vacuum column is most often achieved by using multiple stages of steam jet ejectors. [6]
Many industries, other than the petroleum refining industry, use vacuum distillation on a much a smaller scale.
Laboratory-scale vacuum distillation

Dimethyl sulfoxide boils at 189 °C at atmospheric pressure. Under vacuum, it distills at 70 °C.
Laboratory-scale vacuum distillation, sometimes referred to as low temperature distillation, is used when the liquids to be distilled have high atmospheric boiling points or undergo a chemical change at temperatures near their atmospheric boiling points.[7][8]
Temperature sensitive materials (such as beta carotene) also require vacuum distillation to remove solvents from the mixture without damaging the product.
Some compounds have high boiling points as well as being air sensitive. A simple laboratory vacuum distillation glassware setup can be used, whereby the vacuum is replaced with an inert gas after the distillation is complete. However, this is a less satisfactory system if one desires to collect fractions under a reduced pressure. For better results or for very air sensitive compounds, a Perkin triangle apparatus can be used.
The Perkin triangle uses a series of glass or Teflon valves to allow fractions to be isolated from the still pot without the main body of the distillation setup being removed from either the vacuum or the heat source, and thus can remain in a state of reflux. To do this, the sample collection vessel is first isolated from the vacuum by means of the valves, the vacuum over the sample is then replaced with an inert gas (such as nitrogen or argon) and can then be stoppered and removed. A fresh collection vessel can then be added to the system, evacuated and linked back into the distillation system via the valves to collect a second fraction, and so on, until all fractions have been collected.
Rotary evaporation is a type of vacuum distillation apparatus used to remove bulk solvents from the liquid being distilled. Typically the vacuum in such systems is generated by a water aspirator or a membrane pump.
References
- ↑ 1.0 1.1 Gary, J.H. and Handwerk, G.E. (1984). Petroleum Refining Technology and Economics, 2nd Edition. Marcel Dekker, Inc. ISBN 0-8247-7150-8.
- ↑ 2.0 2.1 Leffler, W.L. (1985). Petroleum refining for the nontechnical person, 2nd Edition. PennWell Books. ISBN 0-87814-280-0.
- ↑ James G, Speight (2006). The Chemistry and Technology of Petroleum, Fourth Edition. CRC Press. 0-8493-9067-2.
- ↑ Kister, Henry Z. (1992). Distillation Design, 1st Edition. McGraw-Hill. ISBN 0-07-034909-6.
- ↑ Karl Kolmetz, Andrew W. Sloley et al (2004), Designing Distillation Columns for Vacuum Service, 11th India Oil and Gas Symposium and International Exhibition, September 2004, Mumbai, India (also published in Hydrocarbon Processing, May 2005)
- ↑ Photo gallery (from website of Graham Manufacturing Company)
- ↑ Vacuum Distillation: New Method for Analyzing Organic Chemicals in a Wide Array of Samples (United States Environmental Protection Agency)
- ↑ What is vacuum distillation? (Argonne National Laboratory's NEWTON Ask-A-Scientist)