User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok |
imported>Milton Beychok |
||
| Line 72: | Line 72: | ||
{| class = "wikitable" align="right" | {| class = "wikitable" align="right" | ||
|+ Athabascan Oil Sands<br>Operating facilities (February 2009)<ref name=QuarterlyReport>[http://www.albertacanada.com/documents/AOSID_QuarterlyUpdate.pdf Alberta Oil Sands Industry, Quarterly Update, February 2, 2009]</ref> | |+ Athabascan Oil Sands<br>Operating facilities (February 2009)<ref name=QuarterlyReport>[http://www.albertacanada.com/documents/AOSID_QuarterlyUpdate.pdf Alberta Oil Sands Industry, Quarterly Update, February 2, 2009]</ref> | ||
! Type of | ! Type of project!! Number<br>of projects!![[U.S. customary units|bbl]]/day<br>of asphalt | ||
|- | |- | ||
| In situ extraction || align="center" | 12 || align="right" | 595,000 | | In situ extraction || align="center" | 12 || align="right" | 595,000 | ||
| Line 82: | Line 82: | ||
| colspan="3"|<small>Note: 1 bbl/day = 158.987 [[Litre|L]]/day = 0.159 m<sup>3</sup>/day</small> | | colspan="3"|<small>Note: 1 bbl/day = 158.987 [[Litre|L]]/day = 0.159 m<sup>3</sup>/day</small> | ||
|} | |} | ||
There are three large natural deposits of asphalt in Alberta, Canada are known as the ''Athabascan oil sands'' and their total surface area is about 54,000 square [[U.S. customary units|miles]] (141,000 square [[Metre|metres]]). The proven reserves of asphalt in those deposits are about 1.7 × 10<sup>12</sup> barrels (270 Gm<sup>3</sup>. About 10% of that is recoverable by current (2009) technology and it is estimated that, with new technologies, the recoverable amount could be about 18-19% which would be about 315 barrels (50 Gm<sup>3</sup>).<ref name=QuarterlyReport/> For comparison, the estimated reserves of petroleum crude oil in Saudi Arabia (as of early 2008) amounts to about 265 × 10<sup>9</sup> barrels (43 Gm<sup>3</sup>).<ref>''Oil& Gas Journal, December 24, 2007''</ref> | There are three large natural deposits of asphalt in Alberta, Canada are known as the ''Athabascan oil sands'' and their total surface area is about 54,000 square [[U.S. customary units|miles]] (141,000 square [[Metre|metres]]). The proven reserves of asphalt in those deposits are about 1.7 × 10<sup>12</sup> barrels (270 Gm<sup>3</sup>). About 10% of that is recoverable by current (2009) technology and it is estimated that, with new technologies, the recoverable amount could be about 18-19% which would be about 315 × 10<sup>9</sup> barrels (50 Gm<sup>3</sup>).<ref name=QuarterlyReport/> For comparison, the estimated reserves of petroleum crude oil in Saudi Arabia (as of early 2008) amounts to about 265 × 10<sup>9</sup> barrels (43 Gm<sup>3</sup>).<ref>''Oil& Gas Journal, December 24, 2007''</ref> | ||
Apshalt is currently being extracted from the Athabascan oil sands and being converted into synthetic petroleum crude oil (referred to as ''[[Syncrude|syncrude]]''. Much of the Athabascan oil sands can be and is being extracted by surface mining techniques. In addition, in situ techniques are being used to extract asphalt from various depths beneath the surface of the oil sands deposits. | Apshalt is currently being extracted from the Athabascan oil sands and being converted into synthetic petroleum crude oil (referred to as ''[[Syncrude|syncrude]]''). Much of the Athabascan oil sands can be and is being extracted by surface mining techniques. In addition, in situ techniques are being used to extract asphalt from various depths beneath the surface of the oil sands deposits. After extraction, the asphalt is converted into synthetic petroleum crude oil in refining facilities that are referred to as ''upgraders''. Currently, about 1,000,000 barrels (159,000 m<sup>3</sup>) of the asphalt are being converted into synthetic crude oil (see adjacent table). Each barrel of oil processed in the upgraders produces 0.86 of a barrel of synthetic crude oil. | ||
The synthetic crude oil is subsequently transported to conventional [[Petroleum refining processes|petroleum refineries]] for processing. Very little, if any, of the extracted asphalt is used for road construction. | |||
== References == | == References == | ||
Revision as of 18:24, 15 February 2009
Petroleum asphalt is a sticky, black and highly viscous liquid or semi-solid that is present in most petroleum crude oils and in some natural deposits. Crude oil is a complex mixture of a great many different hydrocarbons. Petroleum asphalt is defined as that part of crude oil which is separated from the higher-boiling hydrocarbons in crude oil by precipitation upon the addition of lower-boiling hydrocarbon solvents such as propane, pentane, hexane or heptane. The precipitated material consists of asphaltenes which have an average molecular weight of about (800 - 2500 g/mole)[1][2] and exist in the form of flat sheets of polyaromatic condensed rings with short aliphatic chains.[3]
Over the years, petroleum asphalt has been referred to as bitumen, asphaltum or pitch. The terminology varies from country to country and from individual to individual. Asphalt is often confused with coal tar (or coal pitch) derived from the pyrolosis of coal and which has a different chemical structure than asphalt.
When petroleum asphalt is combined with construction aggregate (sand, gravel, crushed stone, etc.) for use in road construction, it has often been referred to as asphaltic concrete, asphaltic cement, bituminous concrete, blacktop or road tar (see Asphalt (paving).
The natural deposits of asphalt (often referred to as tar) include asphaltic lakes such as Bermudez Lake in Venezuela, and Pitch Lake in Trinidad. Other natural deposits include oil sands (often called tar sands) and the two largest deposits of tar sands are such as in Alberta, Canada and the Orinoco Oil Belt area of Venezuela.
History of asphalt
The documented use of naturally occurring asphalt dates back almost to 4000 B.C.:[4][5][6][7]
- 3800 B.C.: Asphalt used for caulking boats made of reeds.
- 3500 B.C.: Asphalt used as cement for jewelry.
- 3000 B.C.: Asphalt used as construction cement by the Sumerians of ancient Mesopotamia (now known as Iraq). Also used to seal a bathing pool or water tank in the city of Mohenjo-Daro in the Indus Valley Civilization located in what is now Pakistan.
- 2500 B.C.: Asphalt and other petroleum oils used in ancient Egypt for embalming mummies. (The Persian word for asphalt is mumiyah which may be related to the English word for mummy).
- 1000 B.C.: Asphalt used for waterproofing by lake dwellers in what is now Switzerland.
- 625 – 650 B.C.: The first recorded use of asphalt as a road-building material was in Babylon during the reigns of King Nabopolassar and his son, King Nebuchadnezzar.
- 500 B.C.: Asphalt mixed with sulfur was used as an incendiary device in the Greek wars. (The word asphalt comes from the Greek word asphaltos, meaning secure in English.)
- 300 B.C. – A.D. 250: Reported occurrences of asphalt and oil seepages in Mesopotamia and the use of liquid asphalt as an illuminant in lamps.
- A.D. 750: First reported use in Italy of asphalt as a coloring material in paintings.
Europeans exploring the Americas discovered natural deposits of asphalt. Writing in 1595, Sir Walter Raleigh described a lake of asphalt on the island of Trinidad, near Venezuela. He used it to recaulk his ships.[5][6]
In the late 1700s and early 1800s, first Pierre-Marie-Jérôme Trésaguet of France, then Thomas Telford and subsequently John Loudon McAdam (both of Scotland) perfected the leveling, draining and contruction of roads using layers of broken stones and gravel. In the period of 1860 – 1880 , to reduce road dust and road maintenance, builders began using hot coal tar to bond the stones together. Such roads became named after McAdam and known as tarmacadam roads, later shortened to tarmac.[5][6][7][8]
In 1870, Belgian chemist Edmond J. DeSmedt laid the first true asphalt pavement in the United States in Newark, New Jersey. He also paved Pennsylvania Avenue in Washington, D.C. in 1876 using 54,000 square yards (45,140 square metres) of sheet asphalt from Pitch Lake in Trinidad.[5][6]
During the early 1900s, coal gasification was being widely used to produce town gas and the by-product tar produced during coal gasification was a readily available product.[8] That tar was extensively used in the construction of tarmacadam (or, more simply, tarmac) roads.
By 1907, asphalt from petroleum refineries had outstripped the use of natural asphalt from Trinidad or elsewhere.[6]. Later in the 1900s, when natural gas replaced town gas, asphalt from petroleum refineries dominated the asphalt paving market from that point on. By the early 1990s, asphalt paving mixture producers in the United States used more than 50 × 106 barrels (7.95 × 106 cubic metres) of petroleum asphalt per year. Of the 2.27 × 106 miles (3.65 × 106 kilometers) of paved road in the United States, 94 percent of them are surfaced with asphalt, including 65 percent of the interstate system. [9]
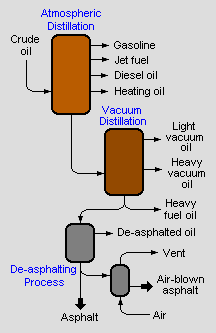
Aphalt production from petroleum crude oil
Asphalt can be separated from the other components in crude oil (such as naphtha, gasoline and diesel) by the process of fractional distillation, usually under vacuum conditions. A better separation can be achieved by further processing of the heavier fractions of the crude oil in a de-asphalting unit, which uses either propane or butane in a supercritical phase to dissolve the lighter molecules which are then separated. Further processing is possible by "blowing" the product: namely reacting it with oxygen. This makes the product harder and more viscous.
Asphalt is typically stored and transported at temperatures around 300 degrees Fahrenheit (150° C). Sometimes diesel oil or kerosene are mixed in before shipping to retain liquidity; upon delivery, these lighter materials are separated out of the mixture. This mixture is often called bitumen feedstock, or BFS. Some dump trucks route the hot engine exhaust through pipes in the dump body to keep the material warm. The backs of tippers carrying asphalt, as well as some handling equipment, are also commonly sprayed with a releasing agent before filling to aid release. Diesel oil is sometimes used as a release agent, although it can mix with and thereby reduce the quality of the asphalt.
Uses of petroleum asphalt
Road construction
The largest use of petroleum asphalt is for making asphaltic concrete for road constuction and accounts for approximately 80% of the asphalt consumed in the United States. The asphalt is used as the binder or glue that holds together the aggregate of sand, gravel, crushed stone, slag or other material.
There are various mixtures of asphalt with other materials that are used in road construction and other paving applications:
- Rolled asphaltic concrete that contains about 95% aggregate and 5% petroleum asphalt binder.
- Mastic asphalt that contains about 90–93% aggregate and 7–10% petroleum asphalt binder.
- Asphalt emulsions that contains about 70% petroleum asphalt and 30% water plus a small amount of chemical additives.
- Cutback asphalt that contains petroleum solvents (referred to as cutbacks).
Only rarely, if ever, are air-blown asphalts used in asphalt-aggregate mixtures for paving purposes.[10]
Roofing shingles
Roofing shingles account for most of the remaining asphalt consumption in the United States.
Other uses
- Asphaltic concrete is widely used for paving vehicle parking lots and aircraft landing and take-off runways in airports around the world
- Canal and reservoir linings as well as dam facings
- Floor tiles
- Battery casings
- Waterproofing of fabrics and various other materials
- Treatment of fence posts and other wooden objects
- Cattle sprays
Asphalt production from oil sands
| Type of project | Number of projects |
bbl/day of asphalt |
|---|---|---|
| In situ extraction | 12 | 595,000 |
| Surface mining | 4 | 1,018,000 |
| Upgrading | 3 | 1,002,000 |
| Note: 1 bbl/day = 158.987 L/day = 0.159 m3/day | ||
There are three large natural deposits of asphalt in Alberta, Canada are known as the Athabascan oil sands and their total surface area is about 54,000 square miles (141,000 square metres). The proven reserves of asphalt in those deposits are about 1.7 × 1012 barrels (270 Gm3). About 10% of that is recoverable by current (2009) technology and it is estimated that, with new technologies, the recoverable amount could be about 18-19% which would be about 315 × 109 barrels (50 Gm3).[11] For comparison, the estimated reserves of petroleum crude oil in Saudi Arabia (as of early 2008) amounts to about 265 × 109 barrels (43 Gm3).[12]
Apshalt is currently being extracted from the Athabascan oil sands and being converted into synthetic petroleum crude oil (referred to as syncrude). Much of the Athabascan oil sands can be and is being extracted by surface mining techniques. In addition, in situ techniques are being used to extract asphalt from various depths beneath the surface of the oil sands deposits. After extraction, the asphalt is converted into synthetic petroleum crude oil in refining facilities that are referred to as upgraders. Currently, about 1,000,000 barrels (159,000 m3) of the asphalt are being converted into synthetic crude oil (see adjacent table). Each barrel of oil processed in the upgraders produces 0.86 of a barrel of synthetic crude oil.
The synthetic crude oil is subsequently transported to conventional petroleum refineries for processing. Very little, if any, of the extracted asphalt is used for road construction.
References
- ↑ Oliver Mullins and Eric Sheu (Editors) (1999). Structure & Dynamics of Asphaltenes, 1st Edition. Springer. ISBN 0-306-45930-2. (See Chapter 1, page 17)
- ↑ Note: There are many other values in the technical literature for the molecular weight of asphaltenes and there does not appear to be a concensus as to which values are more correct.
- ↑ Experimental Investigation of Asphaltene Precipitation From website of the Research Institute of Petroleum Industry in Tehran, Iran.
- ↑ J.G. Speight and Baki Ozum (2002). Petroleum Refining Processes. Marcel Dekker. ISBN 0-8247-0599-8.
- ↑ 5.0 5.1 5.2 5.3 The History of Asphalt From the website of beyondRoads.com.
- ↑ 6.0 6.1 6.2 6.3 6.4 History of Asphalt From the website of the National Asphalt Pavement Association.
- ↑ 7.0 7.1 Online Etymology Dictionary by Douglas Harper
- ↑ 8.0 8.1 Maxwell G. Lay (1999). Handbook of Road Technology, 3rd edition. Taylor&Francis. ISBN 90-5699-157-4.
- ↑ SIC 2951, Asphalt Paving Mixtures and Blocks
- ↑ Air-blown Asphalt: Pilot Plant
- ↑ 11.0 11.1 Alberta Oil Sands Industry, Quarterly Update, February 2, 2009
- ↑ Oil& Gas Journal, December 24, 2007
Yet to do
- Brief section on processing of tar sands as in Canada
- Re-write production process section
- Add more references ... and more books for Bibliography subpage
- Expand section on roofing shingles
Possible References and Books
- Joann A. Wess, Larry D. Olsen and Marie H. Sweeny (2004). Asphalt (bitumen). World Health Organization. ISBN 92-4-153059-6.
- Edwin J. Barth (1962). Asphalt: Science and Technology, 1st Edition. Gordon and Breach Science Publishers. ISBN 0-677-00040-5.
- David S.J. Jones and Peter P.Pujado (Editors) (2006). Handbook of Petroleum Processing, First Edition. Springer. ISBN 1-4020-2819-9.
- OSHA Technical Manual, Asphalt Production From website of the Occupational Safety and Health Administration (OSHA)
- Unconventional Oil: Tar Sands and Shale Oil Part 3 of 6 parts of a series entitled Energy Return on Investment (EROI) on the Web contributed by Professor Charles Hall of the State University of New York (SUNY) and his students (of whom, the authors of this Part 3 were M.C. Herweyer and A. Gupta)