User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok |
imported>Milton Beychok |
||
| Line 29: | Line 29: | ||
== Design and operational variables == | == Design and operational variables == | ||
The selection of whether to use a packed or trayed air stripper is primarily based on which is more cost effective for each specific application. However, in general: | |||
*Trayed strippers can operate efficiently over wider range of liquid flow rates than can a packed stripper, which means that it has lower turndown capability. | |||
*If the liquid contains dispersed solids, the trays in a trayed stripper are easily accessible for cleaning. By contrast, the packing in a packed stripper would have to be completely removed to be cleaned. | |||
*Trayed strippers are seldom designed with cross-sectional diameters less than 60 cm (about 2 feet). | |||
*Packed strippers are preferred for liquids having a tendency to foam since that interferes significantly with the efficiency of trayed strippers. | |||
*Packed towers are less expensive and easier to construct if the liquid is highly corrosive. | |||
*Packed towers are seldom designed with cross-sectional diameters more than 120 cm (about 4 feet). | |||
The are many variables to be considered in the design of an air stripping system. Among them are: | |||
* The volumetric flow rate of the liquid entering the air stripping system. | |||
* The specific volatile components to be removed from the liquid and their concentrations in the liquid entering the stripping system as well as their chemical and physical properties (such as vapor pressure, solubility in the liquid, whether or not they are highly toxic or hazardous, and others). | |||
* The physical properties of the liquid (such as vapor pressure, [[Boiling point|atmospheric boiling point]], [[viscosity]], corrosivity, and others). | |||
* The percentage removal of volatile components from the liquid needed to meet the requirements of any downstream processing of the stripped liquid or to comply with any applicable governmental regulatory requirements for the ultimate disposal of the stripped liquid. | |||
* The limits set by the applicable governmental air pollution regulations as to the pollutants contained in the exit air from the stripping system. | |||
* The temperature of the entering liquid. | |||
The size of the equipment, and particularly the height and diameter, is important in determining the possibility of flow channeling that would reduce the contact area between the liquid and vapor streams. If flow channeling is suspected to be occurring, a redistribution plate is often necessary to, as the name indicates, redistribute the liquid flow evenly to reestablish a higher contact area. | The size of the equipment, and particularly the height and diameter, is important in determining the possibility of flow channeling that would reduce the contact area between the liquid and vapor streams. If flow channeling is suspected to be occurring, a redistribution plate is often necessary to, as the name indicates, redistribute the liquid flow evenly to reestablish a higher contact area. | ||
Revision as of 18:42, 14 September 2010
Air stripping is the transferring of Volatile Organic Compounds (VOC) and/or other volatile components of a liquid into an air stream. It is a chemical engineering process used for the purification of groundwaters and wastewaters containing volatile compounds.[1][2]
Air stripping for the removal of contaminants from groundwaters and wastewaters is especially effective for contaminants that have a high vapor pressure and a low solubility inwater.[1][2]
The compounds removed by air stripping include benzene, toluene, ethylbenzene, and xylene (BTEX) and various solvents (such as trichloroethylene and tetrachloroethylene) found in some wastewaters. Ammonia can also be stripped from wastewaters.
Process description
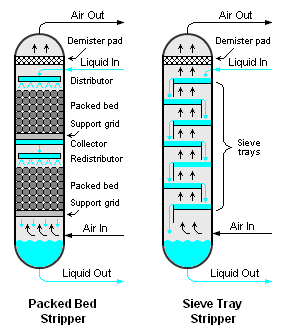
Air stripping is usually accomplished by using either a packed or trayed vertical vessel[3][4][5] as shown in Figure 1. Either type of air stripper involves the downward flow of a volatile-containing liquid (most often, water) and the upward flow of air. The liquid and air are intimately contacted by the packing or the trays in the stripper. The volatile components in the liquid are transferred into the air stream by the series of intimate contacts occurring at each tray as the air flows upward through the stripper.
A properly designed and operated air stripper can remove up to 99.9+ percent of volatile organic compounds (VOC) from groundwaters and wastewaters.
There are many different types of trays, including bubble-cap trays, valve-cap trays and sieve trays. Sieve trays are the most commonly used type in air strippers because they are very simple, less expensive than the other types and adequately efficient for use in air strippers.[6] There are also many types of packing available for packed bed strippers, either randomly packed or structurally packed material as shown in the adjacent photographs.
The stripped liquid leaving the bottom of the stripper is essentially free of volatiles. The air stream leaving the top of the stripper contains the volatiles removed from the liquid and may require some type of emissions mitigation to comply with applicable environmental regulatory limits such as the National Emissions Standards for Hazardous Air Pollutants (HAP) as set by the U.S. Environmental Protection Agency.
When mitigation of the air pollutant emissions from an air stripper is required, there are three technologies available:
- Adsorption in granular activated carbon filters (also known as GAC filters).
- Catalytic oxidation
- Incineration (also known as thermal oxidation)
The relative effectiveness of the above mitigation technologies depend to a great extent on the concentration level of the pollutants in the air stream from an air stripper. Very low pollutant concentrations mean that very large volumes of air must be handled in order to mitigate very small amounts of pollutants. In some cases, it would be prudent to perform air pollution dispersion modeling studies to determine whether or not routing the air stream through a vent stack of a reasonable height would result in compliance with the applicable environmental regulations.
Design and operational variables
The selection of whether to use a packed or trayed air stripper is primarily based on which is more cost effective for each specific application. However, in general:
- Trayed strippers can operate efficiently over wider range of liquid flow rates than can a packed stripper, which means that it has lower turndown capability.
- If the liquid contains dispersed solids, the trays in a trayed stripper are easily accessible for cleaning. By contrast, the packing in a packed stripper would have to be completely removed to be cleaned.
- Trayed strippers are seldom designed with cross-sectional diameters less than 60 cm (about 2 feet).
- Packed strippers are preferred for liquids having a tendency to foam since that interferes significantly with the efficiency of trayed strippers.
- Packed towers are less expensive and easier to construct if the liquid is highly corrosive.
- Packed towers are seldom designed with cross-sectional diameters more than 120 cm (about 4 feet).
The are many variables to be considered in the design of an air stripping system. Among them are:
- The volumetric flow rate of the liquid entering the air stripping system.
- The specific volatile components to be removed from the liquid and their concentrations in the liquid entering the stripping system as well as their chemical and physical properties (such as vapor pressure, solubility in the liquid, whether or not they are highly toxic or hazardous, and others).
- The physical properties of the liquid (such as vapor pressure, atmospheric boiling point, viscosity, corrosivity, and others).
- The percentage removal of volatile components from the liquid needed to meet the requirements of any downstream processing of the stripped liquid or to comply with any applicable governmental regulatory requirements for the ultimate disposal of the stripped liquid.
- The limits set by the applicable governmental air pollution regulations as to the pollutants contained in the exit air from the stripping system.
- The temperature of the entering liquid.
The size of the equipment, and particularly the height and diameter, is important in determining the possibility of flow channeling that would reduce the contact area between the liquid and vapor streams. If flow channeling is suspected to be occurring, a redistribution plate is often necessary to, as the name indicates, redistribute the liquid flow evenly to reestablish a higher contact area.
During operation, monitoring the pressure drop across the column can help to determine the performance of the stripper. A changed pressure drop over a significant range of time can be an indication that the packing may need to be replaced or cleaned.
Typical applications
References
- ↑ 1.0 1.1 Kathleen Sellers (1998). Hazardous Waste Site Remediation, 1st Edition. CRC Press, pages 131 - 134.
- ↑ 2.0 2.1 Air Stripping of VOCs from Water From the website of Jaeger Products, Inc.
- ↑ J.D. Seader and E.J. Henley (2006). Separation Process Principles, 2nd Edition. John Wiley & Sons. ISBN 0-471-46480-5.
- ↑ P. Chattpadhyay (2007). Absorption and Stripping, 1st Edition. Asian Books Pvt.Ltd., pages 2.98 - 2.116. ISBN 81-8412-033-8.
- ↑ E. Roberts Alley (2007). Water Quality Control Handbook, 2nd Edition. McGraw Hill, pages 9.48 - 9.56. ISBN 0-07-146760-2.
- ↑ Note: Sieve trays are simply metal plates perforated with many small holes through which the air flows upward to come into intimate contact with the liquid flowing across the plates. See Figure 1.
External links:
- Process Design Manual for Stripping of Organics Harish M. Shukla and R. Edwin Hicks (1984), U.S. EPA Report EPA-600/2-84-134
- Application of Sieve-Tray Air Strippers to the Treatment of Surfactant-Containing Wastewaters Tohren C. G. Kibbey, Kurt D. Pennell and Kim F. Hayes (2001), AIChE Journal, Vol. 47, No. 6.
- Engineering Design: Air Stripping U.S. Army Corps of Engineers Design Guide 1110-1-3, October 31, 2001
Bibliography:
- American Water Works Association (2003). Water Treatment, 3rd Edition. American Water Works Association. ISBN 1-58321-230-2.
- J. Russell Boulding (Editor) (1996). EPA Environmental Engineering Sourcebook, 1st Edition. CRC Press. ISBN 1-57504-002-6.