Nitroglycerin: Difference between revisions
imported>David E. Volk m (→External Links) |
imported>Howard C. Berkowitz (chest, not chain pain, although I suppose enough chains could bring on chest pain. Caution about THINKING about trying the preparation) |
||
| Line 3: | Line 3: | ||
'''Nitroglycerin''', also called '''glyceryl trinitrate, 1,2,3-Propanetriol trinitrate, nitrin, nitrol or Blasting oil''' | '''Nitroglycerin''', also called '''glyceryl trinitrate, 1,2,3-Propanetriol trinitrate, nitrin, nitrol or Blasting oil''' | ||
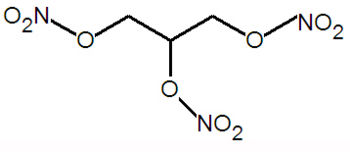
, C<sub>3</sub>H<sub>5</sub>(NO<sub>3</sub>)<sub>3</sub>, is a very unstable, shock-sensitive [[high-explosive]]. Its chemical composition is propane-1,2,3-triyl trinitrate, a [[glycerol]] molecule with three nitro groups substituents on the hydroxy groups. Despite the explosive nature of this compound, it can be used to treat | , C<sub>3</sub>H<sub>5</sub>(NO<sub>3</sub>)<sub>3</sub>, is a very unstable, shock-sensitive [[high-explosive]]. Its chemical composition is propane-1,2,3-triyl trinitrate, a [[glycerol]] molecule with three nitro groups substituents on the hydroxy groups. Despite the explosive nature of this compound, it can be used to treat chest pain. | ||
As a young man, the scientist and inventor [[Alfred Nobel]] (1833-1896)<ref>[http://nobelprize.org/alfred_nobel/biographical/articles/life-work/index.html Alfred Nobel biography]</ref> visited the Paris laboratory of a famous chemist, Professor T. J. Pelouze. There, he met Ascanio Sobrero who, three years earlier, had invented nitroglycerin. Nitroglycerin was then thought much too dangerous to be useful, despite its considerable explosive power, but Nobel became interested in how it might be made safe to handle. Back in his home of Stockholm, he began experiments with his father, but several explosions, including one in 1864 that killed his brother Emil led the authorities that to ban any further experiments within the Stockholm city limits. However, Nobel was not discouraged, he moved his experiments to a barge anchored on Lake Mälaren, and soon began mass production of nitroglycerin. He found that mixing nitroglycerin with a clay (kieselguhr) produced a paste which could be shaped into rods that could be handled and stored relatively safely, and which were suitable for insertion into drilling holes. In 1867, he patented this material, calling it ''dynamite''. | As a young man, the scientist and inventor [[Alfred Nobel]] (1833-1896)<ref>[http://nobelprize.org/alfred_nobel/biographical/articles/life-work/index.html Alfred Nobel biography]</ref> visited the Paris laboratory of a famous chemist, Professor T. J. Pelouze. There, he met Ascanio Sobrero who, three years earlier, had invented nitroglycerin. Nitroglycerin was then thought much too dangerous to be useful, despite its considerable explosive power, but Nobel became interested in how it might be made safe to handle. Back in his home of Stockholm, he began experiments with his father, but several explosions, including one in 1864 that killed his brother Emil led the authorities that to ban any further experiments within the Stockholm city limits. However, Nobel was not discouraged, he moved his experiments to a barge anchored on Lake Mälaren, and soon began mass production of nitroglycerin. He found that mixing nitroglycerin with a clay (kieselguhr) produced a paste which could be shaped into rods that could be handled and stored relatively safely, and which were suitable for insertion into drilling holes. In 1867, he patented this material, calling it ''dynamite''. | ||
==Preparation== | |||
In general terms, the preparation of nitroglycerine involves treating [[glycerine]] with [[nitric acid]] and [[sulfuric acid]]. If one sees a simple recipe for its preparation in more than minute quantities, the procedure is probably both extremely dangerous and incorrect. | |||
Temperature control during the reaction is a critical part of avoiding explosions, as well as purity of the precursors, materials that contact the reactants, and other fine details. Even with manufacturers thoroughly experienced in its production, the actual production line is usually remotely operated, and the building containing it is structured to deflect an explosion into the safest practical direction. | |||
==Medical uses== | ==Medical uses== | ||
Nitroglycerin is used to treat chest pain (angina angina pectoris, also known as ischemic heart disease), and can also be used with other drugs to treat congestive heart failure and heart attacks. It works by relaxing the blood vessels to the heart ([[vasodilation]]), thereby increasing the blood flow and oxygen supply to the heart. It produces a fall in blood pressure and an increase in heart rate. Nitroglycerin reduces chest pain, but it does not cure the underlying causes. | Nitroglycerin is used to treat chest pain (angina angina pectoris, also known as ischemic heart disease), and can also be used with other drugs to treat congestive heart failure and heart attacks. It works by relaxing the blood vessels to the heart ([[vasodilation]]), thereby increasing the blood flow and oxygen supply to the heart. It produces a fall in blood pressure and an increase in heart rate. Nitroglycerin reduces chest pain, but it does not cure the underlying causes. | ||
Revision as of 15:53, 25 September 2008
Nitroglycerin, also called glyceryl trinitrate, 1,2,3-Propanetriol trinitrate, nitrin, nitrol or Blasting oil , C3H5(NO3)3, is a very unstable, shock-sensitive high-explosive. Its chemical composition is propane-1,2,3-triyl trinitrate, a glycerol molecule with three nitro groups substituents on the hydroxy groups. Despite the explosive nature of this compound, it can be used to treat chest pain.
As a young man, the scientist and inventor Alfred Nobel (1833-1896)[1] visited the Paris laboratory of a famous chemist, Professor T. J. Pelouze. There, he met Ascanio Sobrero who, three years earlier, had invented nitroglycerin. Nitroglycerin was then thought much too dangerous to be useful, despite its considerable explosive power, but Nobel became interested in how it might be made safe to handle. Back in his home of Stockholm, he began experiments with his father, but several explosions, including one in 1864 that killed his brother Emil led the authorities that to ban any further experiments within the Stockholm city limits. However, Nobel was not discouraged, he moved his experiments to a barge anchored on Lake Mälaren, and soon began mass production of nitroglycerin. He found that mixing nitroglycerin with a clay (kieselguhr) produced a paste which could be shaped into rods that could be handled and stored relatively safely, and which were suitable for insertion into drilling holes. In 1867, he patented this material, calling it dynamite.
Preparation
In general terms, the preparation of nitroglycerine involves treating glycerine with nitric acid and sulfuric acid. If one sees a simple recipe for its preparation in more than minute quantities, the procedure is probably both extremely dangerous and incorrect.
Temperature control during the reaction is a critical part of avoiding explosions, as well as purity of the precursors, materials that contact the reactants, and other fine details. Even with manufacturers thoroughly experienced in its production, the actual production line is usually remotely operated, and the building containing it is structured to deflect an explosion into the safest practical direction.
Medical uses
Nitroglycerin is used to treat chest pain (angina angina pectoris, also known as ischemic heart disease), and can also be used with other drugs to treat congestive heart failure and heart attacks. It works by relaxing the blood vessels to the heart (vasodilation), thereby increasing the blood flow and oxygen supply to the heart. It produces a fall in blood pressure and an increase in heart rate. Nitroglycerin reduces chest pain, but it does not cure the underlying causes.
The biological effects of nitroglycerin arise because, in the body, the breakdown-products of nitroglycerin include the gas nitric oxide. Nitric oxide is a naturally-produced molecule that is made in the endothelial cells that line blood vessels, and it acts as a vasodilator.
Nitroglycerin (as a medicine) comes in the form of a sublingual tablet, buccal tablet, extended-release (long-acting) capsule, or oral spray. The extended-release formulations are usually taken 3-6 times a day to treat chronic or frequently recurring chest pain. The sublingual tablet and spray are used to relieve acute chest pain that has already started, or to prevent pain before activities that are known to provoke attacks (e.g., exercise or cold weather). Common side-effects include headache, rash, dizziness, upset stomach and flushing (feeling of warmth).[2]
Brand names
- Nitro-Bid®
- Nitrogard®
- Nitroglycerin Slocaps®
- Nitrolingual® Pumpspray
- NitroQuick®
- Nitrostat®
- Nitrotab®
- Nitro-Time®
References
External Links
- Nitroglycerin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank