Pilus: Difference between revisions
imported>David Tribe |
imported>David Tribe |
||
| Line 20: | Line 20: | ||
Pili generate motile force via interactions with the bacteria cytoskeleton MreB which is homologous to eukaryotic actin. The process is akin to the [[myosin]] power stroke. The external termini of the pili adhere to solid substrate, and subsequent pili contraction pulls the bacteria forward, not unlike a grappling hook. | Pili generate motile force via interactions with the bacteria cytoskeleton MreB which is homologous to eukaryotic actin. The process is akin to the [[myosin]] power stroke. The external termini of the pili adhere to solid substrate, and subsequent pili contraction pulls the bacteria forward, not unlike a grappling hook. | ||
===Pili of pathogens=== | ===Pili of pathogens=== | ||
Revision as of 22:13, 19 December 2006
A pilus (Latin for 'hair'; plural : pili) is a hairlike protein structure on the surface of a cell, especially Gram-negative bacteria.
A fimbrium (Latin meaning fringe; plural: fimbriae) is a short pilus that is used to attach the cell to a surface. Fimbriae are either located at the poles of a cell, or are evenly spread over its entire surface. Mutant pathogenic bacteria that lack fimbria cannot adhere to their usual target host cell surfaces and, thus, cannot cause disease.
Bacterial fimbriae can contain lectin-like proteins. These lectins are necessary to adhere to target cells because they can recognize oligosaccharide units such as mannose on the surface of these target cells. However, all types of pili are primarily composed of oligomeric pilin proteins.
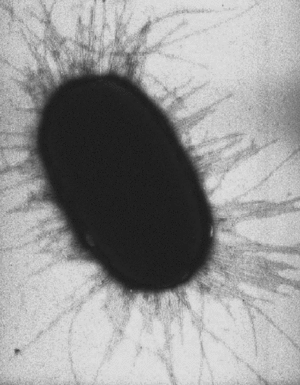
A pilus is typically 9 to 10 nm in diameter, and many of pili structures can exist on the bacteria (see image to right). Some bacterial viruses or bacteriophages attach to receptors on sex pili at the start of their reproductive cycle.
Some forms of pili are encoded by plasmids, for instance fertility factor F encodes F-pili (sex-pili). F-pili are involved in bacterial conjugation and connect the bacterium to another bacterium and build a bridge between the cytoplasm of the cell which is involved in one way transfer of single-strand of DNA and certain proteins. This transfer enables the transfer of plasmids between bacteria. An exchanged plasmid can add new functions to a bacterium, e.g., an antibiotic resistance.
Aggregation and adhesion
The pili encoded by F plasmid in Escherichia coli are involved in formation of cell aggregates typically 2 to 13 cells in number[1]. Numerous genes on the F-plasmid are involved in pilus formation including genes traA, E K B V W U F and G [2].
The pilus allows for the transfer of bacterial DNA from the bacteria with the pilus (donor) to the recipient bacteria. Through this mechanism of gene transfer, advantageous genetic traits can be disseminated amongst a population of bacteria. Not all bacteria have the ability to create sex pili, however sex pili can form between bacteria of different species.
The final shapes of the structures seen in the mature surface biofilms formed by Eschericha coli appear to be determined by pili. Mutants affected in the plasmid specified F-pili form a biofilm of a different structure[3].
Type 4 pili are thin, flexible , 6- to 7-nm fibers displayed by a wide variety of gram-negative bacterial species and members of this class are recognisable because of amino-acid sequence similarities. They have a distinctive method of assembly involving proteolytic processing and N-methylation of the pilin precursor, and they are not implicated in conjugation. They are primarily involved in adhesion to surfaces and as virulence determinants in pathogenic bacteria. In some species they are involved in twitching motility, for instance in Pseudomonas aeruginosa.
Pili generate motile force via interactions with the bacteria cytoskeleton MreB which is homologous to eukaryotic actin. The process is akin to the myosin power stroke. The external termini of the pili adhere to solid substrate, and subsequent pili contraction pulls the bacteria forward, not unlike a grappling hook.
Pili of pathogens
Type 4 pili are produced enteric pathogenic bacteria Vibrio cholerae, enteropathogenic Escherichia coli, and enterotoxigenic E. coli. Bundle forming pili BfpA of enteropathogenic Escherichia coli are Type IV pili that are necessary for adhesion to adhere to epithelial cells. Type 4 pili are virulence factors of Neisseria meningitidis and N. gonorrhoeae. Many bacteria producing Type 4 pili also produce other adhesion factors [4] [5].
Bacterial fimbriae designed to stay with the flow
Liza Gross
The human digestive system houses a diverse colony of beneficial bacteria, but one species—E. coli—can wreak havoc when it colonizes mucous membranes that normally exist unmolested (for example, in the urinary tract). To latch on to cells and establish infection, E. coli uses fimbriae—long, hairlike organelles that project from the bacterium’s surface. Fimbriae consist of interlinking subunits of a single protein called pilin that forms a rigid, coiled helix-shaped rod. Sticky proteins called adhesins cap the tip of the rod and bind to carbohydrate receptors on their host, thus securing bacteria on the host cells as extracellular fluids swirl around them.
A previous study led by Evgeni Sokurenko and Viola Vogel investigated the most common type of E. coli fimbriae. The sticky protein at the tip of these fimbriae is called FimH and binds to a carbohydrate called mannose. They showed that powerful drag forces created by the extracellular fluids don’t carry the bound bacteria away, as one might expect, but instead strengthen their adhesion to their host. The researchers attributed this increased binding to a biphasic “catch bond” mechanism whereby increased drag forces cause the FimH at the tip of the fimbria to switch from a form that binds mannose weakly to a form that binds strongly. Because of this, the bacteria bind best at an optimal force that is high enough to switch FimH to strong binding but not so high that it breaks the strong FimH–mannose bond.
And now, in a new study, the same group of researchers (including first author Manu Forero) set out to determine whether the coiled rod structure of fimbriae affects how the sticky FimH at the tip binds. It had been assumed that fimbrial rods play a largely static structural role, either by extending the tip adhesins’ reach or by resisting electrostatic repulsive forces between bacteria and cell surfaces. But Forero et al. show that the rods function more dynamically, using their mechanical properties to help stabilize the FimH–mannose bond against a turbulent background.
Fimbriae-mediated adhesion was investigated with an atomic force microscope, which uses a cantilever to apply (and measure) forces between its tip and the sample under investigation. Forero et al. outfitted the cantilever tip with mannose, and then used this to touch a fimbriated E. coli cell that was affixed to a glass surface. After mannose bound to the fimbrial FimH, the cantilever retreated from the bacterium at a constant velocity. The researchers determined that, instead of the FimH–mannose bond breaking, the fimbriae stretched out far beyond their original length.
One reason that fimbriae extend could be that the individual pilin subunits of the fimbrial rod are uncoiling. The researchers tested this hypothesis by applying a constant force between the cantilever and fimbria—under which fimbrial length changes slowly. They observed that stepwise jumps in distance corresponded to the expected length of individual subunits unwinding from the coiled shaft one at a time. Thus, the researchers concluded, fimbrial elongation proceeds as subunits uncoil one after another. This was also supported by a mathematical model developed by the researchers to quantify the biophysical forces governing the dynamics of fimbrial uncoiling.
Forero et al. also detected that, after uncoiling at increasing force, the stretched fimbriae re-coil if the pulling force drops. Importantly, while fimbrial uncoiling under high force decreases the tension within the rod, re-coiling under low force increases the tension. Thus, the tensile force within the rod stays within some intermediate level when fimbrial length is stable.
The researchers found that the intermediate force range corresponds to the force level where the FimH–mannose bonds last longest. Lower, coiling forces are too weak to switch bonds to a long-lived state before breaking, and higher, uncoiling forces exceed the catch-bond threshold, shortening the life of the bond. Because E. coli living in the gut or other mucosal surfaces experience constantly changing flow rates and forces, these adjustments should enhance fimbrial attachment under a diverse range of fluid conditions. The correspondence of forces suggests that the mechanical properties of the fimbrial rod and the FimH–mannose complex co-evolved to optimize adhesive stability in fluids.
Citation: Gross L (2006) Bacterial Fimbriae Designed to Stay with the Flow. PLoS Biol 4(9): e314 DOI: 10.1371/journal.pbio.0040314
Published: August 29, 2006
Copyright: © 2006 Public Library of Science. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
Citations
- ↑ Achtman, A. (1975) Mating aggregates in Escherichia coli conjugation . J Bacteriol. August; 123(2): 505–515.
- ↑ Frost, L. S. Ippen-Ihler, K., and Skurray, R. A. (1994). Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiological Reviews 58, p162-210.
- ↑ Reisner, Andreas, Haagensen, Janus A. J. , Schembri, Mark A., Zechner, Ellen L., and Molin, Søren (2003) Development and maturation of Escherichia coli K-12 biofilms Molecular Microbiology 48 (4), pages 933–946
- ↑ Marsh JW, Taylor RK. (1999) Genetic and transcriptional analyses of the Vibrio cholerae mannose-sensitive hemagglutinin type 4 pilus gene locus.
- ↑ J Bacteriol. 1999 Feb;181(4):1110-7.Strom, M. S., and S. Lory. (1993) . Structure-function and biogenesis of the type IV pili. Annu. Rev. Microbiol. 47 pages 565-596.
See also