Percutaneous transluminal coronary angioplasty: Difference between revisions
imported>Robert Badgett |
imported>Robert Badgett No edit summary |
||
| Line 9: | Line 9: | ||
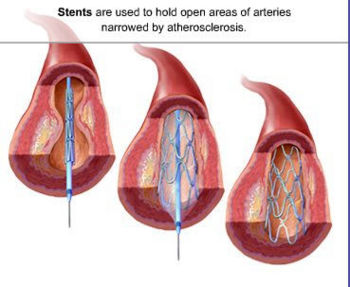
<font><small><tt>{{Image|Coronary stent.jpg|right|350px|</tt></small></font>Insertion of the stent on the delivery catheter, expansion of the stent, and lastly appearance after withdrawal of the delivery catheter.<font><small><tt>}}</tt></small></font> | <font><small><tt>{{Image|Coronary stent.jpg|right|350px|</tt></small></font>Insertion of the stent on the delivery catheter, expansion of the stent, and lastly appearance after withdrawal of the delivery catheter.<font><small><tt>}}</tt></small></font> | ||
Drug-eluting stents further reduce restenosis compared with bare-metal stents<ref name="pmid16971716">{{cite journal |author=Spaulding C, Henry P, Teiger E, ''et al'' |title=Sirolimus-eluting versus uncoated stents in acute myocardial infarction |journal=N. Engl. J. Med. |volume=355 |issue=11 |pages=1093–104 |year=2006 |month=September |pmid=16971716 |doi=10.1056/NEJMoa062006 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=16971716&promo=ONFLNS19 |issn=}}</ref>, but drug-eluting stents may increase the rate of delayed restenoses. Delayed restenosis may be prevented by taking [[aspirin]] combined with [[clopidogrel]].<ref name="pmid17202455"/> The drugs eluted are [[sirolimus]] and [[paclitaxel]]. Sirolimus was first approved in the [[United States]] by the [[Food and Drug Administration]] in 2003.<ref name="urlDevices@FDA11506 ">{{cite web |url=http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=PMA&id=11506 |title=Devices@FDA |author=Anonymous |authorlink= |coauthors= |date=2003 |format= |work= |publisher=Food and Drug Administration |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=2009-05-02}}</ref> Paclitaxel was first approved in the [[United States]] in 2004.<ref name="urlDevices@FDA14019">{{cite web |url=http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=PMA&id=14019 |title=Devices@FDA |author=Anonymous |authorlink= |coauthors= |date=2004 |format= |work= |publisher=Food and Drug Administration |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=2009-05-02}}</ref> | Drug-eluting stents further reduce restenosis compared with bare-metal stents<ref name="pmid16971716">{{cite journal |author=Spaulding C, Henry P, Teiger E, ''et al'' |title=Sirolimus-eluting versus uncoated stents in acute myocardial infarction |journal=N. Engl. J. Med. |volume=355 |issue=11 |pages=1093–104 |year=2006 |month=September |pmid=16971716 |doi=10.1056/NEJMoa062006 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=16971716&promo=ONFLNS19 |issn=}}</ref>, but drug-eluting stents may increase the rate of delayed restenoses. Delayed restenosis may be prevented by taking [[aspirin]] combined with [[clopidogrel]].<ref name="pmid17202455"/> The drugs eluted are [[sirolimus]] and [[paclitaxel]]. Sirolimus was first approved in the [[United States]] by the [[Food and Drug Administration]] in 2003.<ref name="urlDevices@FDA11506 ">{{cite web |url=http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=PMA&id=11506 |title=Devices@FDA |author=Anonymous |authorlink= |coauthors= |date=2003 |format= |work= |publisher=Food and Drug Administration |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=2009-05-02}}</ref> Paclitaxel was first approved in the [[United States]] in 2004.<ref name="urlDevices@FDA14019">{{cite web |url=http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=PMA&id=14019 |title=Devices@FDA |author=Anonymous |authorlink= |coauthors= |date=2004 |format= |work= |publisher=Food and Drug Administration |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=2009-05-02}}</ref> | ||
==Complications== | |||
Ventricular dysrhythmia during PTCA for [[myocardial infarction]] indicates an increased risk for mortality at 3 months.<ref>{{Cite journal | |||
| doi = 10.1001/jama.2009.600 | |||
| volume = 301 | |||
| issue = 17 | |||
| pages = 1779-1789 | |||
| last = Mehta | |||
| first = Rajendra H. | |||
| coauthors = Aijing Z. Starr, Renato D. Lopes, Judith S. Hochman, Petr Widimsky, Karen S. Pieper, Paul W. Armstrong, Christopher B. Granger, for the APEX AMI Investigators | |||
| title = Incidence of and Outcomes Associated With Ventricular Tachycardia or Fibrillation in Patients Undergoing Primary Percutaneous Coronary Intervention | |||
| journal = JAMA | |||
| accessdate = 2009-05-06 | |||
| date = 2009-05-06 | |||
| url = http://jama.ama-assn.org/cgi/content/abstract/301/17/1779 | |||
}}</ref> | |||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 08:49, 6 May 2009
In medicine, percutaneous transluminal coronary angioplasty (PTCA), also called percutaneous coronary intervention (PCI), is a form of myocardial revascularization in which occurs "dilatation of an occluded coronary artery (or arteries) by means of a balloon catheter to restore myocardial blood supply."[1]
PTCA may be a treatment for myocardial infarction and an intravascular stent may or may not be left at the site of the stenosis in order to prevent restenosis.[2]
Stenting reduces the rate of restenosis, but should not be done if the patient cannot take clopidogrel, has extensive stenoses, the stenosis is in a very small coronary artery, or if bypass surgery is planned within a few days.[2]
Drug-eluting stents
Drug-eluting stents further reduce restenosis compared with bare-metal stents[3], but drug-eluting stents may increase the rate of delayed restenoses. Delayed restenosis may be prevented by taking aspirin combined with clopidogrel.[2] The drugs eluted are sirolimus and paclitaxel. Sirolimus was first approved in the United States by the Food and Drug Administration in 2003.[4] Paclitaxel was first approved in the United States in 2004.[5]
Complications
Ventricular dysrhythmia during PTCA for myocardial infarction indicates an increased risk for mortality at 3 months.[6]
References
- ↑ Anonymous (2024), Percutaneous transluminal coronary angioplasty (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ 2.0 2.1 2.2 Keeley EC, Hillis LD (2007). "Primary PCI for myocardial infarction with ST-segment elevation". N. Engl. J. Med. 356 (1): 47-54. DOI:10.1056/NEJMct063503. PMID 17202455. Research Blogging.
- ↑ Spaulding C, Henry P, Teiger E, et al (September 2006). "Sirolimus-eluting versus uncoated stents in acute myocardial infarction". N. Engl. J. Med. 355 (11): 1093–104. DOI:10.1056/NEJMoa062006. PMID 16971716. Research Blogging.
- ↑ Anonymous (2003). Devices@FDA. Food and Drug Administration. Retrieved on 2009-05-02.
- ↑ Anonymous (2004). Devices@FDA. Food and Drug Administration. Retrieved on 2009-05-02.
- ↑ Mehta, Rajendra H.; Aijing Z. Starr, Renato D. Lopes, Judith S. Hochman, Petr Widimsky, Karen S. Pieper, Paul W. Armstrong, Christopher B. Granger, for the APEX AMI Investigators (2009-05-06). "Incidence of and Outcomes Associated With Ventricular Tachycardia or Fibrillation in Patients Undergoing Primary Percutaneous Coronary Intervention". JAMA 301 (17): 1779-1789. DOI:10.1001/jama.2009.600. Retrieved on 2009-05-06. Research Blogging.