Acetaminophen: Difference between revisions
imported>Robert Badgett m (→References) |
imported>Robert Badgett |
||
| Line 18: | Line 18: | ||
== External links == | == External links == | ||
* {{DailyMed}} | * {{DailyMed}} | ||
* | * {{MedMaster}} | ||
* {{DrugBank|DB00316|Acetaminophen}} | |||
Revision as of 07:02, 5 March 2008
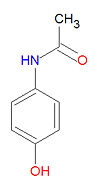
Acetaminophen, also widely called acetaminofen, paracetamol or paracetanol, is an analgesic antipyretic drug widely used for the treatment of headaches, fever and other minor aches and pains. Many cold and flu medications and some prescription analgesics contain acetaminophen. It has no mood altering effects and is not addictive. Acetaminophen inhibits cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) indirectly through a hypothesized yet unknown enzyme called COX-3, in the central nervous system and endothelial cells and thus suppresses the synthesis of prostaglandin and heightens the pain threshold. However, it does not inhibit COX enzymes in the peripheral tissues, and thus has no peripheral anti-inflammatory effects. Although it is sold under hundreds of names, it is popularly known as Tylenol®.
Effectiveness
Toxicity and drug interactions
It may cause liver, blood cell, and kidney damage in a limited number of people. Alcolol intake increases its liver toxicity. The toxic effects of acetaminophen are due to a minor metabolite N-acetyl-p-benzo-quinone imine, which reacts with sulfhydryl groups. At usual doses, it is detoxified by combining with glutathione to produce a non-toxic conjugate that gets excreted by the kidneys.
The anticoagulation effects of acenocoumarol, anisindione, dicumarol and warfarin are increased when taken with acetaminophen. Increased liver toxicity occurs when used in combination with imatinib, isoniazid or alcohol.
One third of patients taking a full doxe (1 gram four times a day) may have their liver function tests rise to three times the upper limit of normal.[1]
References
- ↑ Watkins PB, Kaplowitz N, Slattery JT, et al (2006). "Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial". JAMA 296 (1): 87–93. DOI:10.1001/jama.296.1.87. PMID 16820551. Research Blogging.
External links
- Acetaminophen - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank