Acetaminophen: Difference between revisions
imported>David E. Volk (New page: {{subpages}} [[Image:Acetaminophen structure.jpg|right|thumb|100px|{{#ifexist:Template:Acetaminophen structure.jpg/credit|{{Acetaminophen structure.jpg/credit}}<br/>|}}Acetaminophen (parac...) |
imported>David E. Volk mNo edit summary |
||
| Line 2: | Line 2: | ||
[[Image:Acetaminophen structure.jpg|right|thumb|100px|{{#ifexist:Template:Acetaminophen structure.jpg/credit|{{Acetaminophen structure.jpg/credit}}<br/>|}}Acetaminophen (paracetamol)]] | [[Image:Acetaminophen structure.jpg|right|thumb|100px|{{#ifexist:Template:Acetaminophen structure.jpg/credit|{{Acetaminophen structure.jpg/credit}}<br/>|}}Acetaminophen (paracetamol)]] | ||
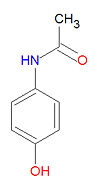
'''Acetaminophen''', also widely called '''acetaminofen''', '''paracetamol''' or '''paracetanol''', is an [[analgesic]] [[antipyretic]] drug widely used for the treatment of headaches, fever and other minor aches and pains. Many [[Common cold|cold]] and [[influenza|flu]] medications and some prescription analgesics contain acetaminophen. It has no mood altering effects and is not addictive. Acetaminophen inhibits [[cyclooxygenase-1 | '''Acetaminophen''', also widely called '''acetaminofen''', '''paracetamol''' or '''paracetanol''', is an [[analgesic]] [[antipyretic]] drug widely used for the treatment of headaches, fever and other minor aches and pains. Many [[Common cold|cold]] and [[influenza|flu]] medications and some prescription analgesics contain acetaminophen. It has no mood altering effects and is not addictive. Acetaminophen inhibits [[cyclooxygenase]]-1 (COX-1) and [[cyclooxygenase]]-2 (COX-2) indirectly through a hypothesized yet unknown enzyme called COX-3, in the [[central nervous system]] and endothelial cells and thus suppresses the synthesis of [[prostaglandin]] and heightens the pain threshold. However, it does not inhibit COX enzymes in the peripheral tissues, and thus has no peripheral anti-inflammatory effects. Although it is sold under hundreds of names, it is popularly known as '''Tylenol'''®. | ||
== Toxicity and drug interactions == | == Toxicity and drug interactions == | ||
Revision as of 16:42, 4 February 2008
Acetaminophen, also widely called acetaminofen, paracetamol or paracetanol, is an analgesic antipyretic drug widely used for the treatment of headaches, fever and other minor aches and pains. Many cold and flu medications and some prescription analgesics contain acetaminophen. It has no mood altering effects and is not addictive. Acetaminophen inhibits cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) indirectly through a hypothesized yet unknown enzyme called COX-3, in the central nervous system and endothelial cells and thus suppresses the synthesis of prostaglandin and heightens the pain threshold. However, it does not inhibit COX enzymes in the peripheral tissues, and thus has no peripheral anti-inflammatory effects. Although it is sold under hundreds of names, it is popularly known as Tylenol®.
Toxicity and drug interactions
It may cause liver, blood cell, and kidney damage in a limited number of people. Alcolol intake increases its liver toxicity. The toxic effects of acetaminophen are due to a minor metabolite N-acetyl-p-benzo-quinone imine, which reacts with sulfhydryl groups. At usual doses, it is detoxified by combining with glutathione to produce a non-toxic conjugate that gets excreted by the kidneys.
The anticoagulation effects of acenocoumarol, anisindione, dicumarol and warfarin are increased when taken with acetaminophen. Increased liver toxicity occurs when used in combination with imatinib, isoniazid or alcohol.
External links
- Acetaminophen - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Drug Bank at http://www.drugbank.ca/cgi-bin/getCard.cgi?CARD=DB00316.txt