User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 2: | Line 2: | ||
A '''chemical reaction''' is a process that leads to the transformation of one set of [[Chemistry|chemical]] substances to another. The one or more substances present at the start of a reaction are called '''''reactants''''' and the one or more substances present at the end of the reaction are called '''''products'''''. The study of chemical reactions is part of the field of [[science]] called [[chemistry]]. | A '''chemical reaction''' is a process that leads to the transformation of one set of [[Chemistry|chemical]] substances to another. The one or more substances present at the start of a reaction are called '''''reactants''''' and the one or more substances present at the end of the reaction are called '''''products'''''. The study of chemical reactions is part of the field of [[science]] called [[chemistry]]. | ||
Chemical reactions can result in [[molecule]]s attaching to each other to form larger molecules, molecules breaking apart to form two or more smaller molecules, or rearrangement of [[atom]]s within or across molecules. Chemical reactions usually involve the making or breaking of [[chemical | Chemical reactions can result in [[molecule]]s attaching to each other to form larger molecules, molecules breaking apart to form two or more smaller molecules, or rearrangement of [[atom]]s within or across molecules. Chemical reactions usually involve the making or breaking of [[Bond (chemical)|chemical bonds]]. | ||
Chemical reactions can be either spontaneous and require no input of [[energy]], or non-spontaneous which often require the input of some type of energy such as [[heat]], [[light]] or [[electricity]]. Classically, chemical reactions are strictly transformations that involve the movement of [[ | Chemical reactions can be either spontaneous<ref name=Rosengarten>[http://www.youtube.com/watch?v=m1nKEz2DPC0 Chemistry Tutorials: Entropy, Enthalpy and Spontaneous Reactions]</ref> and require no input of [[energy]], or non-spontaneous<ref name=Rosengarten/> which often require the input of some type of energy such as [[heat]], [[light]] or [[electricity]]. Classically, chemical reactions are strictly transformations that involve the movement of [[electron]] in the forming and breaking of [[chemical bond]]s. A more general concept of a chemical reaction would include [[nuclear reactions]] and [[Elementary particle|elementary particle reactions]]. | ||
==Energy changes in reactions== | ==Energy changes in reactions== | ||
| Line 37: | Line 37: | ||
*[[Isomerization]], in which a chemical compound undergoes a structural rearrangement without any change in its net atomic composition (see [[stereoisomerism]]) | *[[Isomerization]], in which a chemical compound undergoes a structural rearrangement without any change in its net atomic composition (see [[stereoisomerism]]) | ||
*[[Combination reaction|Direct combination]] or [[Chemical synthesis|synthesis]], in which 2 or more chemical elements or compounds unite to form a more complex product: | *[[Combination reaction|Direct combination]] or [[Chemical synthesis|synthesis]], in which 2 or more [[chemical elements]] or [[Chemical compound|compounds]] unite to form a more complex product: | ||
:[[Nitrogen|N]]<sub>2</sub> + 3 [[Hydrogen|H]]<sub>2</sub> → 2 [[Ammonia|NH<sub>3]]</sub> | :[[Nitrogen|N]]<sub>2</sub> + 3 [[Hydrogen|H]]<sub>2</sub> → 2 [[Ammonia|NH<sub>3]]</sub> | ||
Revision as of 16:36, 11 April 2010
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. The one or more substances present at the start of a reaction are called reactants and the one or more substances present at the end of the reaction are called products. The study of chemical reactions is part of the field of science called chemistry.
Chemical reactions can result in molecules attaching to each other to form larger molecules, molecules breaking apart to form two or more smaller molecules, or rearrangement of atoms within or across molecules. Chemical reactions usually involve the making or breaking of chemical bonds.
Chemical reactions can be either spontaneous[1] and require no input of energy, or non-spontaneous[1] which often require the input of some type of energy such as heat, light or electricity. Classically, chemical reactions are strictly transformations that involve the movement of electron in the forming and breaking of chemical bonds. A more general concept of a chemical reaction would include nuclear reactions and elementary particle reactions.
Energy changes in reactions
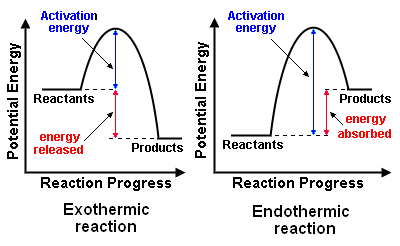
In terms of the energy changes that take place during chemical reactions, a reaction may be either exothermic or endothermic ... terms which were first coined by the French chemist Marcellin Berthelot (1827 − 1907). The meaning of those terms and the difference between them are discussed below and illustrated in the adjacent diagram of the energy profiles for exothermic and endothermic reactions.
Exothermic reactions
Exothermic chemical reactions release energy. The released energy may be in the form of heat, light (e.g., flame), electricity (e.g., battery discharge), sound and shock waves (e.g., explosion) .... either singly or in combinations.
A few examples of exothermic reactions are:
- Mixing of acids and alkalis (releases heat)
- Combustion of fuels (releases heat and light)
Endothermic reactions
Endothermic chemical reactions absorb energy. The energy absorbed may be in various forms just as is the case with exothermic reactions:
A few examples of endothermic reactions are:
- Dissolving ammonium nitrate (NH4NO3) in water (H2O) (absorbs heat and cools the surroundings)
- Electrolysis of water to form hydrogen (H2) and oxygen (O2) gases (absorbs electricity)
- Photosynthesis of chlorophyll plus water plus sunlight to form carbohydrates and oxygen (absorbs light)
Reaction types
The common kinds of classical chemical reactions include:[3]
- Isomerization, in which a chemical compound undergoes a structural rearrangement without any change in its net atomic composition (see stereoisomerism)
- Direct combination or synthesis, in which 2 or more chemical elements or compounds unite to form a more complex product:
- Chemical decomposition in which a compound is decomposed into elements or smaller compounds:
- Single displacement or substitution, characterized by an element being displaced out of a compound by a more reactive element:
- Metathesis or double displacement, in which two compounds exchange ions or bonds to form different compounds:
- Acid-base reactions, broadly characterized as reactions between an acid and a base, can have different definitions depending on the acid-base concept employed. Some of the most common are:
- Arrhenius definition: Acids dissociate in water releasing H3O+ ions; bases dissociate in water releasing OH− ions.
- Brønsted-Lowry definition: Acids are proton (H+) donors; bases are proton acceptors. Includes the Arrhenius definition.
- Lewis definition: Acids are electron-pair acceptors; bases are electron-pair donors. Includes the Brønsted-Lowry definition.
- Redox reactions, in which changes in oxidation numbers of atoms in involved species occur. Those reactions can often be interpreted as transferences of electrons between different molecular sites or species. An example of a redox reaction is:
- 2 S2O32−(aq) + I2(aq) → S4O62−(aq) + 2 I−(aq)
- In which I2 is reduced to I− and S2O32− (thiosulfate anion) is oxidized to S4O62− (tetrathionate anion).
- Combustion, a kind of redox reaction in which any combustible substance combines with an oxidizing element, usually oxygen, to generate heat and form oxidized products. The term combustion is usually used for only large-scale oxidation of whole molecules (i.e., a controlled oxidation of a single functional group is not combustion).
- Disproportionation with one reactant forming two distinct products varying in oxidation state.
- 2 Sn2+ → Sn + Sn4+
- Organic reactions encompass a wide assortment of reactions involving compounds which have carbon as the main element in their molecular structure. The reactions in which an organic compound may take part are largely defined by its functional groups.
References
- ↑ 1.0 1.1 Chemistry Tutorials: Entropy, Enthalpy and Spontaneous Reactions
- ↑ Paul Collison, David Kirby and Averil Macdonald (2002). Nelson Modular Science, Volume 2. Nelson Thorne Ltd.. ISBN 0-7487-6247-7.
- ↑ In the following chemical equations, (aq) indicates an aqueous solution, (g) indicates a gas and (s) indicates a solid. Superscripts with a positive sign (+) indicate an anion and superscripts with a negative sign (−) indicate an anion.