User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok |
||
| Line 10: | Line 10: | ||
{{Image|Catalysis reaction paths.png|right|232px|Add image caption here.}} | {{Image|Catalysis reaction paths.png|right|232px|Add image caption here.}} | ||
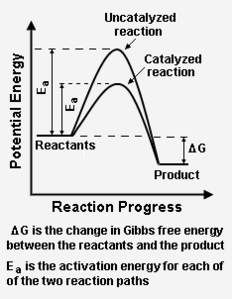
The Collision theory, proposed by Max Trautz[1] and William Lewis in 1916 and 1918, qualitatively explains how chemical reactions occur and why reaction rates differ for different reactions.[2] This theory is based on the idea that reactant particles must collide for a reaction to occur, but only a certain fraction of the total collisions have the energy to connect effectively and cause the reactants to transform into products. This is because only a portion of the molecules have enough energy and the right orientation (or "angle") at the moment of impact to break any existing bonds and form new ones. The minimal amount of energy needed for this to occur is known as activation energy. | |||
<ref>[http://www.chemguide.co.uk/physical/basicrates/catalyst.html#top The Effect of Catalysts on Reaction Rates] Website provided by Jim Clarke, retired Head of Chemistry and then Head of Science at [[Truro School]] in [[Cornwall]], [[United Kingdom]]. | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Revision as of 20:22, 6 April 2010
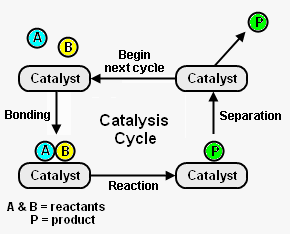
Catalysis is a process that uses a substance to accelerate the rate of a chemical reaction through an uninterrupted and repeated cycle of elementary steps until the last step regenerates the catalyst in its original form. The substance that does this is known as a catalyst. It is usually present in relatively small amounts and none of it is consumed in the process.[1]
Figure 1 depicts the steps in a typical catalysis cycle. As depicted, the reactant molecules A and B are reacted to yield product P. The catalysis cycle starts with the bonding of reactant molecules A and B to the catalyst. A and B then react to yield product P which is also bound to the catalyst. In the last step, the catalyst is regenerated by product P separating from the catalyst. The regenerated catalyst then begins cycle again by bonding with two more reactant molecules. [2]
Many substances can act as catalysts, including: metals, chemical compounds (e.g., metal oxides, sulfides, nitrides), organometallic complexes, and enzymes. Although a catalyst may be a gas, liquid or solid, most catalysts used in industrial chemical reactions are in the form of porous pellets. Since not all parts of a solid catalyst participate in the catalysis cycle, those parts that do participate are referred to as active sites. A single porous pellet may have 1018 active catalytic sites.[1]
The catalysis mechanism
The Collision theory, proposed by Max Trautz[1] and William Lewis in 1916 and 1918, qualitatively explains how chemical reactions occur and why reaction rates differ for different reactions.[2] This theory is based on the idea that reactant particles must collide for a reaction to occur, but only a certain fraction of the total collisions have the energy to connect effectively and cause the reactants to transform into products. This is because only a portion of the molecules have enough energy and the right orientation (or "angle") at the moment of impact to break any existing bonds and form new ones. The minimal amount of energy needed for this to occur is known as activation energy.
<ref>The Effect of Catalysts on Reaction Rates Website provided by Jim Clarke, retired Head of Chemistry and then Head of Science at Truro School in Cornwall, United Kingdom.
References
- ↑ 1.0 1.1 Commission on Physical Sciences, Mathematics, and Applications (CPSMA), National Academies (1992). Catalysis Looks to the Future. National Academies Press. ISBN 0-309-04584-3. Available online at Executive Summary
- ↑ I. Chorkendorff and J. W. Niemantsverdriet (2007). Concepts of Modern Catalysts and Kinetics, 2nd Edition. Wiley-VCH. ISBN 3-527-31672-8.