User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok mNo edit summary |
imported>Milton Beychok No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:Vacuum Column.jpg|right|thumb|183px|{{#ifexist:Template:Vacuum Column.jpg/credit|{{Vacuum Column.jpg/credit}}<br/>|}} | [[Image:Vacuum Column.jpg|right|thumb|183px|{{#ifexist:Template:Vacuum Column.jpg/credit|{{Vacuum Column.jpg/credit}}<br/>|}}Figure 1: A vacuum distillation column in a petroleum refinery.]] | ||
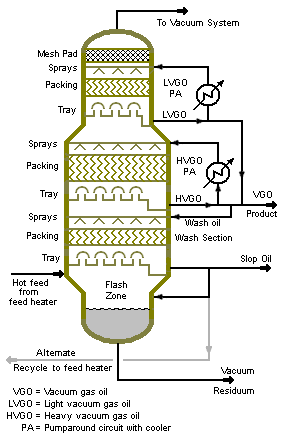
[[Image:Vacuum Column.png|right|thumb|285px|{{#ifexist:Template:Vacuum Column.png/credit|{{Vacuum Column.png/credit}}<br/>|}}Figure 2: Diagram of a vacuum column as used in [[Petroleum refining processes|petroleum refining]].]] | |||
'''Vacuum distillation''' is [[distillation]] of liquids performed at a [[pressure]] lower than [[atmospheric pressure]] to take advantage of the fact that reducing the pressure lowers the [[boiling point]] of liquids. This permits the distillation of liquids that are temperature sensitive and avoids any degradation of such liquids. | |||
== Vacuum distillation in petroleum refining == | |||
[[Petroleum crude oil]] is a complex mixture of hundreds of different [[hydrocarbon]] compounds generally having from 3 to 40 carbon atoms per molecule, although there may be small amounts of hydrocarbons outside that range. In refining the crude oil, it is important not to subject the high [[molecular weight]] components to temperatures above 370 to 380 °C because they will undergo [[thermal cracking]] and form [[petroleum coke]] at temperatures above that. Formation of coke would result in plugging the tubes in the [[furnace]] that heats the feed stream to a column distilling crude oil or the higher molecular weight components of crude oil. Plugging would also occur in the [[piping]] from the furnace to the distillation column as well as in the column itself. | |||
The refining of crude oil begins with distilling the incoming crude oil in a so-called [[Petroleum refining processes#The crude oil distillation unit|''atmospheric distillation column'']] operating at pressures slightly above atmospheric pressure. The constraint imposed by limiting the column inlet temperature to no more than 370 to 380 °C yields a residual oil from the bottom of the atmospheric distillation column that consists entirely of high molecular weight hydrocarbons that boil above 370 to 380 °C. | |||
In order to distill the residual oil from the atmospheric distillation column, it is necessary to perform the distillation at [[absolute pressure]]s as low as 10 to 40 [[mmHg]] so as to limit the operating temperature to less than 370 to 380 °C. | |||
Figure 1 is a photograph of a large vacuum distillation column in a petroleum refinery and Figure 2 is a process diagram of a petroleum refinery vacuum distillation column that depicts the internals of the column. | |||
The 10 to 40 mmHg absolute pressure in a vacuum distillation column increases the volume of vapor formed per volume of liquid distilled. The result is that such columns have much larger diameters than columns than columns operating at atmospheric pressure. Distillation columns such those in Figures 1 and 2, may have diameters of 15 meters or more. | |||
The vacuum distillation column internals must provide good vapor-liquid contacting while, at the same time, maintaining a very low pressure increase from the top of the column top to the bottom. | |||
(discuss how low delta P achieved) (discuss use of ejectors) | |||
[[Image:Vacuum distillation of DMSO at 70C.jpg|right|thumb|350px|{{#ifexist:Template:Vacuum distillation of DMSO at 70C.jpg/credit|{{Vacuum distillation of DMSO at 70C.jpg/credit}}<br/>|}}Laboratory distillation apparatus]] | [[Image:Vacuum distillation of DMSO at 70C.jpg|right|thumb|350px|{{#ifexist:Template:Vacuum distillation of DMSO at 70C.jpg/credit|{{Vacuum distillation of DMSO at 70C.jpg/credit}}<br/>|}}Laboratory distillation apparatus]] | ||
[[Image:Perkins triangle distillation apparatus.png|right|thumb |200px| Add caption here]] | [[Image:Perkins triangle distillation apparatus.png|right|thumb |200px| Add caption here]] | ||
Revision as of 05:31, 9 February 2008
Vacuum distillation is distillation of liquids performed at a pressure lower than atmospheric pressure to take advantage of the fact that reducing the pressure lowers the boiling point of liquids. This permits the distillation of liquids that are temperature sensitive and avoids any degradation of such liquids.
Vacuum distillation in petroleum refining
Petroleum crude oil is a complex mixture of hundreds of different hydrocarbon compounds generally having from 3 to 40 carbon atoms per molecule, although there may be small amounts of hydrocarbons outside that range. In refining the crude oil, it is important not to subject the high molecular weight components to temperatures above 370 to 380 °C because they will undergo thermal cracking and form petroleum coke at temperatures above that. Formation of coke would result in plugging the tubes in the furnace that heats the feed stream to a column distilling crude oil or the higher molecular weight components of crude oil. Plugging would also occur in the piping from the furnace to the distillation column as well as in the column itself.
The refining of crude oil begins with distilling the incoming crude oil in a so-called atmospheric distillation column operating at pressures slightly above atmospheric pressure. The constraint imposed by limiting the column inlet temperature to no more than 370 to 380 °C yields a residual oil from the bottom of the atmospheric distillation column that consists entirely of high molecular weight hydrocarbons that boil above 370 to 380 °C.
In order to distill the residual oil from the atmospheric distillation column, it is necessary to perform the distillation at absolute pressures as low as 10 to 40 mmHg so as to limit the operating temperature to less than 370 to 380 °C.
Figure 1 is a photograph of a large vacuum distillation column in a petroleum refinery and Figure 2 is a process diagram of a petroleum refinery vacuum distillation column that depicts the internals of the column.
The 10 to 40 mmHg absolute pressure in a vacuum distillation column increases the volume of vapor formed per volume of liquid distilled. The result is that such columns have much larger diameters than columns than columns operating at atmospheric pressure. Distillation columns such those in Figures 1 and 2, may have diameters of 15 meters or more.
The vacuum distillation column internals must provide good vapor-liquid contacting while, at the same time, maintaining a very low pressure increase from the top of the column top to the bottom. (discuss how low delta P achieved) (discuss use of ejectors)