User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok |
imported>Milton Beychok |
||
| Line 21: | Line 21: | ||
===Density of solid objects by x-ray diffraction crytallography=== | ===Density of solid objects by x-ray diffraction crytallography=== | ||
For some [[crystal|crystalline]] materials, density can be calculated using this equation | For some [[crystal|crystalline]] materials, density can be calculated using this equation which is based the [[molecular mass]] of the material and on information provided by [[x-ray diffraction|x-ray crystallography]]: | ||
:<math>\mbox{density} = \frac{M \cdot N} {L \cdot a \cdot b \cdot c} </math> | :<math>\mbox{density} = \frac{M \cdot N} {L \cdot a \cdot b \cdot c} </math> | ||
| Line 29: | Line 29: | ||
:'''''M''''' is molecular mass | :'''''M''''' is molecular mass | ||
:'''''N''''' is the number of [[ion]s, [[atom]]s or [[molecule]]s in a [[unit cell]] | :'''''N''''' is the number of [[ion]]s, [[atom]]s or [[molecule]]s in a [[unit cell]] | ||
:'''''L''''' is [[Loschmidt]] or [[Avogadro's constant]] | :'''''L''''' is [[Loschmidt]] or [[Avogadro's constant]] | ||
Revision as of 17:37, 17 June 2008
Measurement of density
For a homogeneous solid object, the mass divided by the volume gives the density. The mass is normally measured with an appropriate weighing scale or balance and the volume may be measured directly (from the geometry of the object) or by the displacement of a liquid.
A very simple and commonly used instrument for directly measuring the density of a liquid is the hydrometer. A less common device for measuring fluid density is a pycnometer and a similar device for measuring the absolute density of a solid is a gas pycnometer.
Another instrument used to determine the density of a liquid or a gas is the digital density meter, based on the oscillating U-tube principle.
Density of irregular shaped solid object heavier than water
One of the many statements attributed to the Archimedes principle is that an object immersed in a fluid apparently loses weight by an amount equal to the weight of the fluid displaced.[1][2][3] That principle makes it possible to determine of the density of a solid object that is denser than water and is so irregular in shape that its volume cannot be measured directly.
First, the object's dry weight in air, , is measured. Next, the weight of the object while submerged in water, , is measured. Then the submerged object is removed from the water, the excess surface water is removed and the wet weight in air, , is measured.[4] The density of the object, , is then obtained from:
and the volumetric porosity fraction, can be obtained from:
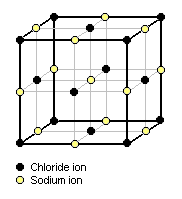
Density of solid objects by x-ray diffraction crytallography
For some crystalline materials, density can be calculated using this equation which is based the molecular mass of the material and on information provided by x-ray crystallography:
where:
- M is molecular mass
- L is Loschmidt or Avogadro's constant
- a, b, c are the lattice parameters
For example, [5] the cubic lattice structure of crystalline sodium chloride (NaCl) is depicted in the Figure 1.
- ↑ California State University Dominguez Hills
- ↑ Murat Bengisu. Engineering Ceramics, First Edition. Springer, pages 363-366. ISBN 3-540-67687-2.
- ↑ ASTM C373-88 Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity and Apparent Specific Gravity of Fired Whiteware
- ↑ American Society of Composites (1994). American Society of Composites, Ninth International Conference Proceedings. CRC Press, page 539. 1-56676-220-0.
- ↑ Alexander Findlay (1942). Practical Physical Density. Longman, Greens and Company, 164-167.