User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 1: | Line 1: | ||
<u>'''This needs a lot of editing and rewording.'''</u><BR><BR> | <u>'''This needs a lot of editing and rewording.'''</u><BR><BR> | ||
'''Gasoline''' or '''petrol''' is derived from [[petroleum crude oil]]. It is a blended mixture of 200 | '''Gasoline''' or '''petrol''' is derived from [[petroleum crude oil]]. It is a blended mixture of more than 200 [[hydrocarbon]] [[liquid]]s ranging from those containing 4 [[carbon]] [[atom]]s to those containing 11 or 12 carbon atoms. It has an initial [[boiling point]] at [[atmospheric pressure]] of about 35 °[[Celsius|C]] (95 °[[Fahrenheit|F]]) and a final boiling point of about 200 °C (395 °F).<ref name=FAQ>[http://www.faqs.org/faqs/autos/gasoline-faq/part4/ Gasoline FAQ - Part2 of 4], Bruce Hamilton, Industrial Research Ltd. (IRL), a [[Crown Research Institute]] of [[New Zealand]].</ref><ref>{{cite book|author=Gary, J.H. and Handwerk, G.E.|title=Petroleum Refining Technology and Economics|edition=2nd Edition|publisher=Marcel Dekker, Inc.|pages=page 8|year=1984|id=ISBN 0-8247-7150-8}}</ref><ref>{{cite book|author=James Speight|title=Synthetic Fuels Handbook|edition=1st Edition|publisher=McGraw-Hill|pages=pages 92-93|year=2008|id=ISBN 0-07-149023-X}}</ref>Gasoline is used as fuel for the [[internal combustion engine]]s in automotive vehicles as well in some small airplanes. | ||
Some gasoline marketed in parts of the United States and elswhere also contains a significant amount of [[ethanol]]. | Some gasoline marketed in parts of the United States and elswhere also contains a significant amount of [[ethanol]]. | ||
| Line 25: | Line 25: | ||
* In many geographical areas, the amount of gasoline produced during the summer season (i.e., the season of the greatest demand for automotive gasoline) varies significantly from the amount produced during the winter season. | * In many geographical areas, the amount of gasoline produced during the summer season (i.e., the season of the greatest demand for automotive gasoline) varies significantly from the amount produced during the winter season. | ||
The various refinery process streams that are blended together to obtain the end-product gasolines all have different characteristics. Some of the most important gasoline blending components are: | |||
*''Reformate'': produced in a [[Catalytic reforming|catalytic reformer]] and has a high content of [[aromatic hydrocarbons]] and a low content very of [[olefinic hydrocarbons]] ([[alkene]]s). | |||
*''Catalytically cracked gasoline'': produced in a [[Fluid catalytic cracking|fluid catalytic cracker]] and has a high content of olefinic hydrocarbons a moderate amount of aromatic hydrocarbons. | |||
*''Hydrocrackate'': produced from a [[Hydrocracking|hydrocracker]] and a moderate content of aromatic hydrocarbons. | |||
*''Alkylate'': produced in an [[Alkylation process|alkylation unit]] has a high content of highly branched paraffinic hydrocarbons such as isooctane. | |||
*''Isomerate'': produced by [[Catalytic isomerization|isomerising] the pentane and hexane their branched isomers. | |||
Currently many countries set tight limits on gasoline [[aromatic]]s in general, [[benzene]] in particular, and olefin (alkene) content. This is increasing the demand for high octane pure paraffin (alkane) components, such as alkylate, and is forcing refineries to add processing units to reduce the benzene content. | Currently many countries set tight limits on gasoline [[aromatic]]s in general, [[benzene]] in particular, and olefin (alkene) content. This is increasing the demand for high octane pure paraffin (alkane) components, such as alkylate, and is forcing refineries to add processing units to reduce the benzene content. | ||
Revision as of 23:06, 21 March 2009
This needs a lot of editing and rewording.
Gasoline or petrol is derived from petroleum crude oil. It is a blended mixture of more than 200 hydrocarbon liquids ranging from those containing 4 carbon atoms to those containing 11 or 12 carbon atoms. It has an initial boiling point at atmospheric pressure of about 35 °C (95 °F) and a final boiling point of about 200 °C (395 °F).[1][2][3]Gasoline is used as fuel for the internal combustion engines in automotive vehicles as well in some small airplanes.
Some gasoline marketed in parts of the United States and elswhere also contains a significant amount of ethanol.
In Canada and the United States, the word "gasoline" is commonly used and it is often shortened to simply "gas" although it is a liquid rather than a gas. In fact, gasoline dispensing facilities are referred to as "gas stations".
Most current or former Commonwealth countries use the term "petrol" and dispensing facilities are referred to as "petrol stations". The term "petrogasoline" is also used sometimes.
In aviation, "mogas" (short for "motor gasoline") is used to distinguish automotive vehicle fuel from aviation fuel known as "avgas".
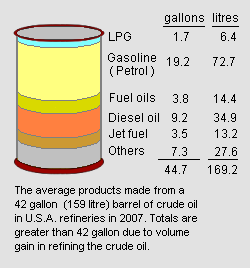
Amount of gasoline produced from a barrel of crude oil
It is very difficult to quantify the amount of gasoline produced from a given amount of petroleum crude oil for a number of reasons:
- There are quite literally hundreds of different crude oil sources worldwide and each crude oil has its own unique mixture of thousands of hydrocarbons and other materials.
- There are also hundreds of crude oil refineries worldwide and each of them is designed to process a specific crude oil or a specific set of crude oils. Furthermore, each refinery has its own unique configuration of petroleum refining processes that produces its own unique set of gasoline blend components.
- There are a great many different gasoline specifications that have been mandated by various local, state or national govermental agencies.
- In many geographical areas, the amount of gasoline produced during the summer season (i.e., the season of the greatest demand for automotive gasoline) varies significantly from the amount produced during the winter season.
The various refinery process streams that are blended together to obtain the end-product gasolines all have different characteristics. Some of the most important gasoline blending components are:
- Reformate: produced in a catalytic reformer and has a high content of aromatic hydrocarbons and a low content very of olefinic hydrocarbons (alkenes).
- Catalytically cracked gasoline: produced in a fluid catalytic cracker and has a high content of olefinic hydrocarbons a moderate amount of aromatic hydrocarbons.
- Hydrocrackate: produced from a hydrocracker and a moderate content of aromatic hydrocarbons.
- Alkylate: produced in an alkylation unit has a high content of highly branched paraffinic hydrocarbons such as isooctane.
- Isomerate: produced by [[Catalytic isomerization|isomerising] the pentane and hexane their branched isomers.
Currently many countries set tight limits on gasoline aromatics in general, benzene in particular, and olefin (alkene) content. This is increasing the demand for high octane pure paraffin (alkane) components, such as alkylate, and is forcing refineries to add processing units to reduce the benzene content.
Gasoline can also contain some other organic compounds: such as organic ethers (deliberately added), plus small levels of contaminants, in particular sulfur compounds such as disulfides and thiophenes. Some contaminants, in particular thiols and hydrogen sulfide, must be removed because they cause corrosion in engines.
Octane rating
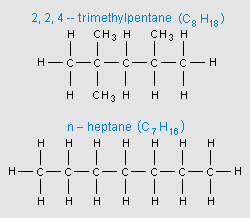
An important characteristic of gasoline is its octane rating, which is a measure of how resistant gasoline is to the abnormal combustion phenomenon known as pre-detonation (also known as knocking, pinging, spark knock, and other names). Deflagration is the normal type of combustion. Octane rating is measured relative to a mixture of 2,2,4-trimethylpentane (an isomer of octane) and n-heptane. There are a number of different conventions for expressing the octane rating; therefore, the same fuel may be labeled with a different number, depending upon the system used.
The octane rating became important in the search for higher output powers from aero engines in the late 1930s and the 1940s as it allowed higher compression ratios to be used.
Additives
Tetra-ethyl lead (TEL)
The mixture known as gasoline, when used in high compression internal combustion engines, has a tendency to autoignite (detonation) causing a damaging "engine knocking" (also called "pinging") noise. Early research into this effect was led by A.H. Gibson and Harry Ricardo in England and Thomas Midgley and Thomas Boyd in the United States. The discovery that lead additives modified this behavior led to the widespread adoption of the practice in the 1920s and therefore more powerful higher compression engines. The most popular additive was tetra-ethyl lead. However, with the discovery of the environmental and health damage caused by the lead, and the incompatibility of lead with catalytic converters found on virtually all newly sold US automobiles since 1975, this practice began to wane (encouraged by many governments introducing differential tax rates) in the 1980s. Most countries are phasing out leaded fuel; different additives have replaced the lead compounds. The most popular additives include aromatic hydrocarbons, ethers and alcohol (usually ethanol or methanol). In the US, where lead had been blended with gasoline (primarily to boost octane levels) since the early 1920s, standards to phase out leaded gasoline were first implemented in 1973 - due in great part to studies conducted by Philip J. Landrigan. In 1995, leaded fuel accounted for only 0.6% of total gasoline sales and less than 2,000 short tons of lead per year. From January 1, 1996, the Clean Air Act banned the sale of leaded fuel for use in on-road vehicles. Possession and use of leaded gasoline in a regular on-road vehicle now carries a maximum $10,000 fine in the US. However, fuel containing lead may continue to be sold for off-road uses, including aircraft, racing cars, farm equipment, and marine engines.[4] The ban on leaded gasoline led to thousands of tons of lead not being released in the air by automobiles. Similar bans in other countries have resulted in lowering levels of lead in people's bloodstreams.[5][6]
A side effect of the lead additives was protection of the valve seats from erosion. Many classic cars' engines have needed modification to use lead-free fuels since leaded fuels became unavailable. However, "Lead substitute" products are also produced and can sometimes be found at auto parts stores. These were scientifically tested and some were approved by the Federation of British Historic Vehicle Clubs at the UK's Motor Industry Research Association (MIRA) in 1999. http://www.fbhvc.co.uk/fuel/index.htm
Gasoline, as delivered at the pump, also contains additives to reduce internal engine carbon buildups, improve combustion, and to allow easier starting in cold climates.
In some parts of South America, Asia, Eastern Europe and the Middle East, leaded gasoline is still in use. Leaded gasoline was phased out in sub-Saharan Africa effective 1 January, 2006. A growing number of countries have drawn up plans to ban leaded gasoline in the near future.
MMT
Methylcyclopentadienyl manganese tricarbonyl (MMT) has been used for many years in Canada and recently in Australia to boost octane. It also helps old cars designed for leaded fuel run on unleaded fuel without need for additives to prevent valve problems.
US Federal sources state that MMT is suspected to be a powerful neurotoxin and respiratory toxin,[7] and a large Canadian study concluded that MMT impairs the effectiveness of automobile emission controls and increases pollution from motor vehicles.[8]
In 1977 use of MMT was banned in the US by the Clean Air Act until the Ethyl Corporation could prove that the additive would not lead to failure of new car emissions-control systems. As a result of this ruling, the Ethyl Corporation began a legal battle with the EPA, presenting evidence that MMT was harmless to automobile emissions-control systems. In 1995 the US Court of Appeals ruled that the EPA had exceeded its authority, and MMT became a legal fuel additive in the US. MMT is nowadays manufactured by the Afton Chemical Corporation division of Newmarket Corporation.[9]
Oxygenates
Oxygenate blending adds oxygen to the fuel in oxygen-bearing compounds such as MTBE, ETBE and ethanol, and so reduces the amount of carbon monoxide and unburned fuel in the exhaust gas, thus reducing smog. In many areas throughout the US oxygenate blending is mandated by EPA regulations to reduce smog and other airborne polutants. For example, in Southern California, fuel must contain 2% oxygen by weight, resulting in a mixture of 5.6% ethanol in gasoline. The resulting fuel is often known as reformulated gasoline (RFG) or oxygenated gasoline. The federal requirement that RFG contain oxygen was dropped May 6, 2006 because the industry had developed VOC-controlled RFG that did not need additional oxygen.[10]
MTBE use is being phased out in some states due to issues with contamination of ground water. In some places, such as California, it is already banned. Ethanol and to a lesser extent the ethanol derived ETBE are a common replacements. Especially since ethanol derived from biomatter such as corn, sugar cane or grain is frequent, this will often be referred to as bio-ethanol. A common ethanol-gasoline mix of 10% ethanol mixed with gasoline is called gasohol or E10, and an ethanol-gasoline mix of 85% ethanol mixed with gasoline is called E85. The most extensive use of ethanol takes place in Brazil, where the ethanol is derived from sugarcane. In 2004, over 3.4 billion US gallons (2.8 billion imp gal/13 million m³) of ethanol was produced in the United States for fuel use, mostly from corn, and E85 is slowly becoming available in much of the United States. Unfortunately many of the relatively few stations vending E85 are not open to the general public.[11] The use of bioethanol, either directly or indirectly by conversion of such ethanol to bio-ETBE, is encouraged by the European Union Directive on the Promotion of the use of biofuels and other renewable fuels for transport. However since producing bio-ethanol from fermented sugars and starches involves distillation, ordinary people in much of Europe cannot legally ferment and distill their own bio-ethanol at present (unlike in the US where getting a BATF distillation permit has been easy since the 1973 oil crisis.)
Ethanol
In the United States, ethanol is sometimes added to gasoline but sold without an indication that it is a component. Chevron, 76, Shell, and several other brands market ethanol-gasoline blends.Template:Fact
In several states, ethanol is added by law to a minimum level which is currently 5.9%. Most fuel pumps display a sticker stating that the fuel may contain up to 10% ethanol, an intentional disparity which allows the minimum level to be raised over time without requiring modification of the literature/labeling. The bill which was being debated at the time the disclosure of the presence of ethanol in the fuel was mandated has recently passed. This law (Energy Policy Act of 2005) will require all auto fuel to contain at least 10% ethanol. Many call this fuel mix gasohol.
In the EU, 5% ethanol can be added within the common gasoline spec (EN 228). Discussions are ongoing to allow 10% blending of ethanol. Most countries (fuel distributors) today do not add so much ethanol.Template:Fact Most gasoline (petrol) sold in Sweden has 5% ethanol added.
In Brazil, the Brazilian National Agency of Petroleum, Natural Gas and Biofuels (ANP) requires that gasoline for automobile use has 23% of ethanol added to its composition.
Stability
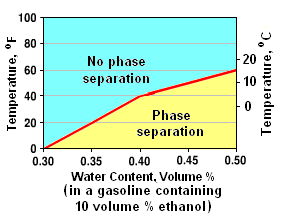
Temperatures and associated water contents at which a blend of gasoline and 10 volume % ethanol separates.[12]
When gasoline is left for a period of time, gums and varnishes may build up and precipitate in the gasoline, causing "stale fuel." This will cause gums to build up in the fuel tank, lines, and carburetor or fuel injection components making it harder to start the engine. Motor gasoline may be stored up to 60 days in an approved container. If it is to be stored for a longer period of time, a fuel stabilizer may be used. This will extend the life of the fuel to about 1–2 years, and keep it fresh for the next uses. Fuel stabilizer is commonly used for small engines such as lawnmower and tractor engines to promote quicker and more reliable starting. Users have been advised to keep gasoline containers and tanks more than half full and properly capped to reduce air exposure, to avoid storage at high temperatures,[13] to run an engine for ten minutes to circulate the stabilizer through all components prior to storage, and to run the engine at intervals to purge stale fuel from the carburetor.[14]
Gummy, sticky resin deposits result from oxidative degradation of gasoline. This degradation can be prevented through the use of antioxidants such as phenylenediamines, alkylenediamines (diethylenetriamine, triethylenetetramine, etc), and alkylamines (diethylamine, tributylamine, ethylamine). Other useful additives include gum inhibitors such as N-substituted alkylaminophenols and colour stabilizers such as N-(2-aminoethyl)piperazine, N,N-diethylhydroxylamine, and triethylenetetramine.[15]
Improvements in refinery techniques have generally reduced the reliance on the catalytically or thermally cracked stocks most susceptible to oxidation.[16] Gasoline containing acidic contaminants such as naphthenic acids can be addressed with additives including strongly basic organo-amines such as N,N-diethylhydroxylamine, preventing metal corrosion and breakdown of other antioxidant additives due to acidity. Hydrocarbons with a bromine number of 10 or above can be protected with the combination of unhindered or partially hindered phenols and oil soluble strong amine bases such as monoethanolamine, N-(2-aminoethyl)piperazine, cyclohexylamine, 1,3-cyclohexane-bis(methylamine), 2,5-dimethylaniline, 2,6-dimethylaniline, diethylenetriamine and triethylenetetramine.[15]
References
- ↑ Gasoline FAQ - Part2 of 4, Bruce Hamilton, Industrial Research Ltd. (IRL), a Crown Research Institute of New Zealand.
- ↑ Gary, J.H. and Handwerk, G.E. (1984). Petroleum Refining Technology and Economics, 2nd Edition. Marcel Dekker, Inc., page 8. ISBN 0-8247-7150-8.
- ↑ James Speight (2008). Synthetic Fuels Handbook, 1st Edition. McGraw-Hill, pages 92-93. ISBN 0-07-149023-X.
- ↑ U.S. Environmental Protection Agency (1996-01-29). EPA Takes Final Step in Phaseout of Leaded Gasoline. Press release.
- ↑ Lourdes Schnaas, Stephen J. Rothenberg, María-Fernanda Flores, Sandra Martínez, Carmen Hernández, Erica Osorio,1 and Estela Perroni (2004). "Blood Lead Secular Trend in a Cohort of Children in Mexico City (1987–2002)" (Open-access full-text reprint). Environ. Health. Perspect. 112 (10): 1110–1115. DOI:10.1289/ehp.6636. PMID 15238286. Research Blogging.
- ↑ Paulina Pino, Tomás Walter; Manuel J. Oyarzún A3, Matthew J. Burden; Betsy Lozoff (2004). "Rapid Drop in Infant Blood Lead Levels during the Transition to Unleaded Gasoline Use in Santiago, Chile". Archives of Environmental Health: An International Journal 59 (4): 182–187. DOI:10.3200/AEOH.59.4.182-187. Research Blogging.
- ↑ Comments of the Gasoline Additive MMT. Retrieved on 2008-08-10.
- ↑ Final Report: Effects of MMT in Gasoline on Emissions from On-Road Motor Vehicles in Canada (PDF). Canadian Vehicle Manufacturers’ Association, and Association of International Automobile Manufacturers of Canada (2002-11-11).
- ↑ History of mmt. Afton Chemical. Retrieved on 2008-02-22.
- ↑ Removal of Reformulated Gasoline Oxygen Content Requirement (national) and Revision of Commingling Prohibition to Address Non-0xygenated Reformulated Gasoline (national). U.S. Environmental Protection Agency (2006-02-22).
- ↑ Alternative Fueling Station Locator. U.S. Department of Energy.
- ↑ E10 & E85 and Other Alternate Fuels Bruce Bauman, American Petroleum Institute(API)
- ↑ Fuel storage practices.
- ↑ PER Notebook.
- ↑ 15.0 15.1 Template:Patent
- ↑ Template:Patent
External links
References
- David S.J. Jones and Peter P.Pujado (Editors) (2006). Handbook of Petroleum Processing, First Edition. Springer. ISBN 1-4020-2819-9.
- John McKetta (Editor) (1992). Petroleum Processing Handbook. CRC Press. ISBN 0-8247-8681-5.
- Gasoline FAQ - Part2 of 4, Bruce Hamilton, Industrial Research Ltd. (IRL), a Crown Research Institute of New Zealand.
- The Relation Between Gasoline Quality, Octane Number and the Environment, Rafat Assi, National Project Manager of Jordan’s Second National Communications on Climate Change, Presented at Jordan National Workshop on Lead Phase-out, United Nations Environment Programme, July 2008, Amman, Jordan.
- Questions and Answers Relating to the Review of the Existing Fuel Quality Regulations, New Zealand Ministry of Economic Development, December 2005.
- Where Does My Gasoline Come from?, U.S. Department of Energy, Energy Information Administration, April 2008.
- Otto Cycle (About the internal combustion engine four-stroke cycle invented by Nicolaus A. Otto)
- CRS Report For Congress "Boutique Fuels" and Reformulated Gasoline: Harmonization of Fuel Standards (May 10, 2006) , Brent D. Yacobucci, Congressional Research Service, Library of Congress