User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok |
imported>Milton Beychok |
||
| Line 23: | Line 23: | ||
(Note: In distillation terminology, when a liquid or vapor mixture attains a higher concentration of some component, it is referred to as being ''enriched'', ''rich'' or ''richer'' in that component.) | (Note: In distillation terminology, when a liquid or vapor mixture attains a higher concentration of some component, it is referred to as being ''enriched'', ''rich'' or ''richer'' in that component.) | ||
Heat is required to provide the multiple occurrences of partial vaporization | Heat is required to provide the multiple occurrences of partial vaporization in a distillation column. The required heat is applied to the bottom of a distillation column in a number of ways, the most common being the transfer of heat from a [[reboiler]]. | ||
Similarly, cooling is required to provide the multiple occurrences of partial condensation that also occur in a distillation column. The required cooling is most usually provided by a [[condenser]] used to cool and condense the overhead vapor into a liquid and then returning part of the cool condensed liquid to the top of the column as [[reflux]]. | Similarly, cooling is required to provide the multiple occurrences of partial condensation that also occur in a distillation column. The required cooling is most usually provided by a [[condenser]] used to cool and condense the overhead vapor into a liquid and then returning part of the cool condensed liquid to the top of the column as [[reflux]]. | ||
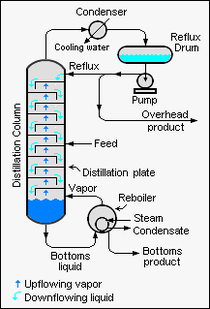
Figure 1 is a schematic diagram of a continuous binary distillation column such as described above. The overhead condenser may be water-cooled or air-cooled. The bottoms reboiler may be a [[heat exchanger] heated by steam or hot oil, or it might be a fuel-fired [[furnace]]. The location of the feed entry can vary from one design to another and is selected to provide optimum results (see McCabe-Thiele method). | Figure 1 is a schematic diagram of a continuous binary distillation column such as described above. The overhead condenser may be water-cooled or air-cooled. The bottoms reboiler may be a [[heat exchanger]] heated by steam or hot oil, or it might be a fuel-fired [[furnace]]. The location of the feed entry can vary from one design to another and is selected to provide optimum results (see McCabe-Thiele method). | ||
A continuous distillation column is kept in a [[steady state]] or approximate steady state. In the context of continuous distillation, that means thet the feed rate, output product rates, [[reflux]] rate, heating and cooling rates, [[temperature]]s, pressures, and compositions at every point within the column are essentially kept constant during operation. It also means that the column is material-balanced and heat-balanced (i.e., the material inputs equal the material outputs, and the heat inputs equal the heat outputs). | A continuous distillation column is kept in a [[steady state]] or approximate steady state. In the context of continuous distillation, that means thet the feed rate, output product rates, [[reflux]] rate, heating and cooling rates, [[temperature]]s, pressures, and compositions at every point within the column are essentially kept constant during operation. It also means that the column is material-balanced and heat-balanced (i.e., the material inputs equal the material outputs, and the heat inputs equal the heat outputs). | ||
Revision as of 18:04, 19 July 2008
Continuous distillation is an ongoing process in which a liquid mixture of two or more miscible components is continuously fed into the process and continuously separated into two or more products by preferentially boiling the more volatile components out of the mixture. [1][2][3][4]
Large-scale, continuous distillation is used widely in the chemical process industries where large quantities of liquids have to be distilled, as in petroleum refining, natural gas processing, petrochemical production, coal tar processing and the liquefaction of gases such as hydrogen, oxygen, nitrogen, and helium).
Industrial distillation is typically performed in large, vertical cylindrical columns (see adjacent photograph) known as "distillation towers" or "distillation columns" with diameters ranging from about 65 centimeters to 11 meters and heights ranging from about 6 meters to 60 meters or more.
Distillation is one of the important unit operations of chemical engineering. If the feed contains more than two components, it is referred to as multi-component distillation and, if it contains only two components, it is referred to as binary distillation.
The process
When a liquid mixture is heated so that it boils, the evolved vapor will have a higher concentration of the more volatile (i.e., lower boiling point) components than the liquid mixture from which it evolved. Conversely, when a vapor mixture is cooled, the less volatile components tend to condense in a greater proportion than the more volatile components.
That is what happens in a distillation column. A liquid mixture is fed into the distillation column. On entering the column, the heated feed is partially vaporized and rises up the column. However, as it rises, it cools and partially condenses so that, while part of it continues up as vapor, the condensed portion is enriched in the less volatile component(s) and descends. As the vapor continues to flow upward, it undergoes partial condensation a number of times and each time becomes richer in the more volatile component(s).
The part of the feed liquid that did not vaporize on entering the column, flows downward and is heated until it is partially vaporized. The resulting vapor flows upward and the residual liquid is enriched in the less volatile component(s) and descends. As the liquid continues to flow downward, it undergoes partial vaporization a number of times and each time becomes richer in the less volatile component(s).
The overhead vapor that exits the top of the column is rich in the more volatile component(s) of the column feed and the bottoms liquid which exits the bottom of the column is rich in the less volatile component(s) of the column feed.
(Note: In distillation terminology, when a liquid or vapor mixture attains a higher concentration of some component, it is referred to as being enriched, rich or richer in that component.)
Heat is required to provide the multiple occurrences of partial vaporization in a distillation column. The required heat is applied to the bottom of a distillation column in a number of ways, the most common being the transfer of heat from a reboiler.
Similarly, cooling is required to provide the multiple occurrences of partial condensation that also occur in a distillation column. The required cooling is most usually provided by a condenser used to cool and condense the overhead vapor into a liquid and then returning part of the cool condensed liquid to the top of the column as reflux.
Figure 1 is a schematic diagram of a continuous binary distillation column such as described above. The overhead condenser may be water-cooled or air-cooled. The bottoms reboiler may be a heat exchanger heated by steam or hot oil, or it might be a fuel-fired furnace. The location of the feed entry can vary from one design to another and is selected to provide optimum results (see McCabe-Thiele method).
A continuous distillation column is kept in a steady state or approximate steady state. In the context of continuous distillation, that means thet the feed rate, output product rates, reflux rate, heating and cooling rates, temperatures, pressures, and compositions at every point within the column are essentially kept constant during operation. It also means that the column is material-balanced and heat-balanced (i.e., the material inputs equal the material outputs, and the heat inputs equal the heat outputs).
- ↑ Kister, Henry Z. (1992). Distillation Design, 1st Edition. McGraw-Hill. ISBN 0-07-034909-6.
- ↑ King, C.J. (1980). Separation Processes. McGraw Hill. 0-07-034612-7.
- ↑ Perry, Robert H. and Green, Don W. (2007). Perry's Chemical Engineers' Handbook, 8th Edition. McGraw-Hill. ISBN 0-07-142294-3.
- ↑ McCabe, W., Smith, J. and Harriott, P. (2004). Unit Operations of Chemical Engineering, 7th Edition. McGraw Hill. ISBN 0-07-284823-5.