User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok |
imported>Milton Beychok |
||
| Line 35: | Line 35: | ||
==Process flow diagram and description== | ==Process flow diagram and description== | ||
The Claus reaction to convert H<sub>2</sub> into elemental sulfur requires the presence of one [[mole (unit)|mole]] of SO<sub>2</sub> for each two moles of H<sub>2</sub>S: | The Claus reaction to convert H<sub>2</sub>S into elemental sulfur requires the presence of one [[mole (unit)|mole]] of SO<sub>2</sub> for each two moles of H<sub>2</sub>S: | ||
::2H<sub>2</sub>S + SO<sub>2</sub> → 3S + 2H<sub>2</sub>O | ::(1) 2H<sub>2</sub>S + SO<sub>2</sub> → 3S + 2H<sub>2</sub>O | ||
To provide that ratio of components, the first step in the Claus process is the combustion of one-third of the H<sub>2</sub>S in the feed gas: | To provide that ratio of components, the first step in the Claus process is the combustion of one-third of the H<sub>2</sub>S in the feed gas: | ||
::H<sub>2</sub>S + 1.5 O<sub>2</sub> → SO<sub>2</sub> + H<sub>2</sub>O | ::(2) H<sub>2</sub>S + 1.5 O<sub>2</sub> → SO<sub>2</sub> + H<sub>2</sub>O | ||
Combining equations (1) and (2), the overall process reaction is: | |||
::(3) 2H<sub>2</sub>S + O<sub>2</sub> → 2S + 2H<sub>2</sub>O | |||
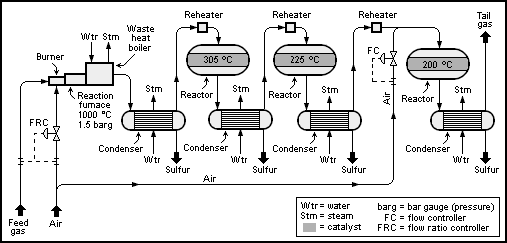
As shown in the [[Process flow diagram|schematic diagram]] below, the feed gas to a Claus process unit is burned in a reaction furnace using sufficient combustion air to burn only one-third of the H<sub>2</sub>S it contains. That is accomplished by using control valve (called a ''flow ratio controller'') to provide the required ratio of combustion air to feed gas. | |||
The reaction furnace pressure and temperature is maintained at about 1.5 [[Bar (unit)|barg]] and 1,000 °C. At those conditions, the Claus reaction occurs thermally in the reaction furnace (i.e., without requiring any catalyst). About 70% of the H<sub>2</sub>S in the feed gas is thermally converted into elemental sulfur in the reaction furnace. | |||
The hot reaction product gas, containing gaseous sulfur, is used to produce [[steam]] in a boiler (called a ''waste heat boiler'') which results in cooling the gases. The gas is then further cooled and [[Condensation|condensed]] in a [[heat exchanger]] while producing additional steam. The condensed liquid sulfur is separated from the remaining unreacted gas in the outlet end of the [[condenser]] and sent to product storage. | |||
The separated gas is then reheated and enters the first catalytic reactor maintained at an average temperature of about 305 °C where about 20% of the H<sub>2</sub>S in the feed gas is thermally converted into elemental sulfur. The outlet product gas from the first reactor is cooled in another condenser while also producing steam. Again, the condensed liquid sulfur is separated from the remaining unreacted gas in the outlet end of the [[condenser]] and sent to product storage. | |||
The separated gas from the second condenser is sent to another reheater and the sequence of gas reheat, catalytic reaction, condensation and separation of liquid sulfur from unreacted gas is repeated for the second and third reactors at successively lower reactor temperatures. About 5% and 3% of the H<sub>2</sub>S in the feed gas is thermally converted into elemental sulfur in the second reactor and third reactors, respectively. For a well-designed and operated Claus sulfur recovery plant having three catalytic reactors (as shown in the flow diagram), an overall conversion of at least 98% can be achieved. In fact, the latest modern designs can achieve 99 | |||
The | The remaining gas separated from the last condenser is referred to as ''tail gas'' and is either burned in an [[incinerator]] or further desulfurized in a ''tail gas treatment unit'' (TGTU). | ||
[[Image:Claus Sulfur Recovery.png|frame|center|453px|{{#ifexist:Template:Claus Sulfur Recovery.png/credit|{{Claus Sulfur Recovery.png/credit}}<br/>|}}Schematic diagram of a Claus sulfur recovery unit.]] | |||
== | ==Miscellaneous items to be edited and used in the article== | ||
Gases containing ammonia, such as the gas from the refinery's sour water stripper (SWS), or hydrocarbons are converted in the burner muffle. Sufficient air is injected into the muffle for the complete combustion of all hydrocarbons and ammonia. | |||
To reduce the process gas volume or obtain higher combustion temperatures, the air requirement can also be covered by injecting pure oxygen. Several technologies utilizing high-level and low-level oxygen enrichment are available in industry, which requires the use of a special burner in the reaction furnace for this process option. | |||
The catalytic | The Claus reaction continues in the catalytic step with activated alumina or titanium dioxide, and serves to boost the sulfur yield. | ||
Where an incineration or tail-gas treatment unit (TGTU) is added downstream of the Claus plant, only two catalytic stages are usually installed. | |||
Several methods of reheating are used in industry: | Several methods of reheating are used in industry: | ||
| Line 84: | Line 82: | ||
The typically recommended operating temperature of the first catalyst stage is 315°C to 330°C (bottom bed temperature). The high temperature in the first stage also helps to hydrolyze COS and CS<sub>2</sub>, which is formed in the furnace and would not otherwise be converted in the modified Claus process. | The typically recommended operating temperature of the first catalyst stage is 315°C to 330°C (bottom bed temperature). The high temperature in the first stage also helps to hydrolyze COS and CS<sub>2</sub>, which is formed in the furnace and would not otherwise be converted in the modified Claus process. | ||
Care must be taken to ensure that each bed is operated above the dewpoint of sulfur. | |||
Before storage, liquid sulfur streams from the process gas cooler, the sulfur condensers and from the final sulfur separator are routed to the degassing unit, where the gases (primarily H<sub>2</sub>S) dissolved in the sulfur are removed. | Before storage, liquid sulfur streams from the process gas cooler, the sulfur condensers and from the final sulfur separator are routed to the degassing unit, where the gases (primarily H<sub>2</sub>S) dissolved in the sulfur are removed. | ||
Gases with an [[Hydrogen sulfide|H<sub>2</sub>S]] content of over 25% are suitable for the recovery of sulfur in straight-through Claus plants while alternate configurations such as a split-flow set up or feed and air preheating can be used to process leaner feeds.<ref>Gas Processors Association Data Book, 10th Edition, Volume II, Section 22</ref> | Gases with an [[Hydrogen sulfide|H<sub>2</sub>S]] content of over 25% are suitable for the recovery of sulfur in straight-through Claus plants while alternate configurations such as a split-flow set up or feed and air preheating can be used to process leaner feeds.<ref>Gas Processors Association Data Book, 10th Edition, Volume II, Section 22</ref> | ||
Revision as of 00:07, 5 August 2008
The Claus process is a catalytic chemical process for converting gaseous hydrogen sulfide (H2S) into elemental sulfur.[1] The process is commonly referred to as a sulfur recovery unit (SRU) and is very widely used to produce sulfur from the hydrogen sulfide found in raw natural gas and from the by-product gases containing hydrogen sulfide derived from refining petroleum crude oil and other industrial facilities.
There are many hundreds of Claus sulfur recovery units in operation worldwide. In fact, the vast majority of the 66,000,000 metric tons of sulfur produced worldwide in 2006 was by-product sulfur from petroleum refining and natural gas processing plants.[2][3]
Feed gas composition
Claus unit feed gases have a wide range of compositions. Most of the feed gases are originate from absorption processes using various solvents to extract hydrogen sulfide from the by-product gases of petroleum refining, natural gas processing, tar sands processing, coal gasification and other industries. The absorption processes used for that purpose include amine gas treating, Rectisol and Selexol to name a few.
In addition to hydrogen sulfide extracted from by-product gases by an absorption process, petroleum refineries also derive hydrogen sulfide from the steam distillation of wastewaters containing dissolved hydrogen sulfide. Those wastewaters are referred to as sour water and the steam distillation of those wastewaters is referred to as sour water stripping.
The table below provides typical analyses of the Claus feed gases obtained from amine gas treating and from sour water stripping:
| Component | Mol % | Wt % | Component | Mol % | Wt % |
|---|---|---|---|---|---|
| From an amine process: | From a sour water stripper: | ||||
| H2S | 82.1 | 80.8 | H2S | 26.7 | 40.2 |
| CO2 | 11.9 | 15.1 | CO2 | 2.6 | 5.1 |
| NH3 | nil | nil | NH3 | 39.4 | 29.7 |
| H2O | 4.0 | 2.1 | H2O | 31.3 | 25.0 |
| HC | 2.0 | 2.0 | HC | nil | nil |
Gases with an H2S content of over 25% are suitable for the recovery of sulfur in straight-through Claus plants while alternate configurations such as a split-flow set up or feed and air preheating can be used to process leaner feeds.[4]
The amount of hydrogen sulfide derived from sour water sripping in a petroleum refinery is very much less than than is derived from the refinery's sour water stripper.
Process flow diagram and description
The Claus reaction to convert H2S into elemental sulfur requires the presence of one mole of SO2 for each two moles of H2S:
- (1) 2H2S + SO2 → 3S + 2H2O
To provide that ratio of components, the first step in the Claus process is the combustion of one-third of the H2S in the feed gas:
- (2) H2S + 1.5 O2 → SO2 + H2O
Combining equations (1) and (2), the overall process reaction is:
- (3) 2H2S + O2 → 2S + 2H2O
As shown in the schematic diagram below, the feed gas to a Claus process unit is burned in a reaction furnace using sufficient combustion air to burn only one-third of the H2S it contains. That is accomplished by using control valve (called a flow ratio controller) to provide the required ratio of combustion air to feed gas.
The reaction furnace pressure and temperature is maintained at about 1.5 barg and 1,000 °C. At those conditions, the Claus reaction occurs thermally in the reaction furnace (i.e., without requiring any catalyst). About 70% of the H2S in the feed gas is thermally converted into elemental sulfur in the reaction furnace.
The hot reaction product gas, containing gaseous sulfur, is used to produce steam in a boiler (called a waste heat boiler) which results in cooling the gases. The gas is then further cooled and condensed in a heat exchanger while producing additional steam. The condensed liquid sulfur is separated from the remaining unreacted gas in the outlet end of the condenser and sent to product storage.
The separated gas is then reheated and enters the first catalytic reactor maintained at an average temperature of about 305 °C where about 20% of the H2S in the feed gas is thermally converted into elemental sulfur. The outlet product gas from the first reactor is cooled in another condenser while also producing steam. Again, the condensed liquid sulfur is separated from the remaining unreacted gas in the outlet end of the condenser and sent to product storage.
The separated gas from the second condenser is sent to another reheater and the sequence of gas reheat, catalytic reaction, condensation and separation of liquid sulfur from unreacted gas is repeated for the second and third reactors at successively lower reactor temperatures. About 5% and 3% of the H2S in the feed gas is thermally converted into elemental sulfur in the second reactor and third reactors, respectively. For a well-designed and operated Claus sulfur recovery plant having three catalytic reactors (as shown in the flow diagram), an overall conversion of at least 98% can be achieved. In fact, the latest modern designs can achieve 99
The remaining gas separated from the last condenser is referred to as tail gas and is either burned in an incinerator or further desulfurized in a tail gas treatment unit (TGTU).
Miscellaneous items to be edited and used in the article
Gases containing ammonia, such as the gas from the refinery's sour water stripper (SWS), or hydrocarbons are converted in the burner muffle. Sufficient air is injected into the muffle for the complete combustion of all hydrocarbons and ammonia.
To reduce the process gas volume or obtain higher combustion temperatures, the air requirement can also be covered by injecting pure oxygen. Several technologies utilizing high-level and low-level oxygen enrichment are available in industry, which requires the use of a special burner in the reaction furnace for this process option.
The Claus reaction continues in the catalytic step with activated alumina or titanium dioxide, and serves to boost the sulfur yield.
Where an incineration or tail-gas treatment unit (TGTU) is added downstream of the Claus plant, only two catalytic stages are usually installed.
Several methods of reheating are used in industry:
- Hotgas bypass: which involves mixing the two process gas streams from the process gas cooler (cold gas) and the bypass (hot gas) from the first pass of the Wasteheat boiler.
- Indirect Steam reheaters: the gas can also be heated with high pressure steam in a heat exchanger.
- Gas/Gas Exchangers: whereby the cooled gas from the process gas cooler is indirectly heated from the hot gas coming out of an upstream catalytic reactor in a gas-to-gas exchanger.
- Direct-fired Heaters: fired reheaters utilizing acid gas or fuel gas, which is burned substoichiometrically to avoid oxygen breakthrough which can damage Claus catalyst.
The typically recommended operating temperature of the first catalyst stage is 315°C to 330°C (bottom bed temperature). The high temperature in the first stage also helps to hydrolyze COS and CS2, which is formed in the furnace and would not otherwise be converted in the modified Claus process.
Care must be taken to ensure that each bed is operated above the dewpoint of sulfur.
Before storage, liquid sulfur streams from the process gas cooler, the sulfur condensers and from the final sulfur separator are routed to the degassing unit, where the gases (primarily H2S) dissolved in the sulfur are removed.
Gases with an H2S content of over 25% are suitable for the recovery of sulfur in straight-through Claus plants while alternate configurations such as a split-flow set up or feed and air preheating can be used to process leaner feeds.[5]
Process performance
Using two catalytic stages, the process will typically yield over 97% of the sulfur in the input stream. Over 2.6 tons of steam will be generated for each ton of sulfur yield.
History
First invented over 100 years ago, the Claus process has become the industry standard.
The process was invented by Carl Friedrich Claus, a chemist working in England. A British patent was issued to him in 1883. The process was later significantly modified by a German company called I.G.Farbenindustrie A.G.[6]
References
- ↑ Gary, J.H. and Handwerk, G.E. (1984). Petroleum Refining Technology and Economics, 2nd Edition. Marcel Dekker, Inc.. 0824771508.
- ↑ Sulfur production report by the United States Geological Survey
- ↑ Discussion of recovered byproduct sulfur
- ↑ Gas Processors Association Data Book, 10th Edition, Volume II, Section 22
- ↑ Gas Processors Association Data Book, 10th Edition, Volume II, Section 22
- ↑ Bibliographic Citation