Leucine zipper: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk m (small picture text) |
mNo edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
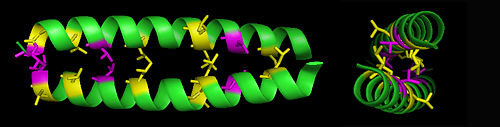
[[Image:GCN4 Leucine Zipper.jpg|right|thumb|500px|<small>The leucine zipper structure of [[GCN4]].<ref>{{cite journal|title=X-Ray Structure of the GCN4 Leucine Zipper, a Two-Stranded Coiled Coil|author=E.K. O'Shea,J.D.Klemm,P.S.Kim and T.Alber|journal=Science|volume=254|pages=539|year=1991}}</ref> The leucines (yellow) and valines (magenta) form a hydrophobic zipper interaction.</small>]] | [[Image:GCN4 Leucine Zipper.jpg|right|thumb|500px|<small>The leucine zipper structure of [[GCN4]].<ref>{{cite journal|title=X-Ray Structure of the GCN4 Leucine Zipper, a Two-Stranded Coiled Coil|author=E.K. O'Shea,J.D.Klemm,P.S.Kim and T.Alber|journal=Science|volume=254|pages=539|year=1991}}</ref> The leucines (yellow) and valines (magenta) form a hydrophobic zipper interaction.</small>]] | ||
'''Leucine zippers''' are a commonly | '''Leucine zippers''' are a commonly occurring [[structural motif]] in [[protein structure]]s, particularly in [[DNA]]-binding proteins, in which the amino acid [[leucine]] is repeated every seven amino acids within an [[alpha-helix]] structure. Additional leucines or [[valine]]s may be present every 3rd or 4th position between the leucines. This sequence of amino acids creates an <math>\alpha</math>-helix with a very hydrophobic face, so that two such proteins can form what is termed a coiled-coil structure. Both homodimer and heterodimer leucine zippers occur naturally. | ||
== References == | == References == | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:00, 11 September 2024
Leucine zippers are a commonly occurring structural motif in protein structures, particularly in DNA-binding proteins, in which the amino acid leucine is repeated every seven amino acids within an alpha-helix structure. Additional leucines or valines may be present every 3rd or 4th position between the leucines. This sequence of amino acids creates an -helix with a very hydrophobic face, so that two such proteins can form what is termed a coiled-coil structure. Both homodimer and heterodimer leucine zippers occur naturally.
References
- ↑ E.K. O'Shea,J.D.Klemm,P.S.Kim and T.Alber (1991). "X-Ray Structure of the GCN4 Leucine Zipper, a Two-Stranded Coiled Coil". Science 254: 539.