Hydrophilic: Difference between revisions
imported>David E. Volk (→Soaps) |
mNo edit summary |
||
| (7 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
'''Hydrophilic''' | '''Hydrophilic''', from Greek (hydros) "water" (philia) "friendship". A chemical term indicating that a chemical compound or group is water soluble. Its opposite is the term [[hydrophobic]], meaning ''water-repelling'' or ''water-fearing''. Lipophilic and lipophobic hold the same meaning as hydrophobic and hydrophilic. Hydrophilic and hydrophobic properties of chemicals play a significant role in chemical reactions, detergents and more importantly in the organization living organisms. Hydrophilic parts of chemicals often carry an electric charge or a polar moiety, and they are attracted to each other by charge-charge or dipole-dipole interactions. Hydrophobic parts of molecules are usually alkanes or aromatic in nature, and they are attracted to each other mostly by [[van der Waals forces]]. | ||
== Solubility == | == Solubility == | ||
| Line 6: | Line 6: | ||
== Soaps == | == Soaps == | ||
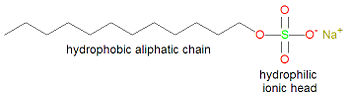
{{Image|Sodium lauryl sulfate.jpg|right|350px|'''Sodium lauryl sulfate (sodium dodecyl sulfate), a typical detergent molecule'''}} | |||
[[Soap]]s and [[detergent]]s<ref>Soaps are sodium salts of fatty acids, which tend to precipitate in hard water containing magnesium or calcium ions, leaving a scum. Detergents contain other hydrophlic groups, like sulfate, to reduce scum buildup.</ref> owe their action to a combination of hydrophilic and hydrophobic ends on the same molecule. | [[Soap]]s and [[detergent]]s<ref>Soaps are sodium salts of fatty acids, which tend to precipitate in hard water containing magnesium or calcium ions, leaving a scum. Detergents contain other hydrophlic groups, like sulfate, to reduce scum buildup.</ref> owe their action to a combination of hydrophilic and hydrophobic ends on the same molecule. | ||
The hydrophic ends are | The hydrophic ends are attracted to each other, and grease, for example, by hydrophobic interactions. The hydrophilic ends of soap molecules are attracted to water. The result is that the hydrophobic aliphatic ends of the soap molecules surround the dirt or grease, with the hydrophilic ends pointing outwards, thus making the grease removable as a spherical [[micelle]]. The grease is said to be [[emulsified]], that is, held in solution in a solvent in which it is not normally soluble. | ||
== Cells == | == Cells == | ||
All biological [[cell]]s | All biological [[cell]]s maintain micelle bilayers that separate the cell from the outside world. These bilayers are formed when the hydrophilic ends of long fatty acids are attacked to each other, leaving the hydrophobic ends displayed to the outside world. Polar chemicals then need to be escorted into and out of the cell by transporter proteins or specialized pores to get through this hydrophobic region. | ||
== References == | |||
{{reflist}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:00, 30 August 2024
Hydrophilic, from Greek (hydros) "water" (philia) "friendship". A chemical term indicating that a chemical compound or group is water soluble. Its opposite is the term hydrophobic, meaning water-repelling or water-fearing. Lipophilic and lipophobic hold the same meaning as hydrophobic and hydrophilic. Hydrophilic and hydrophobic properties of chemicals play a significant role in chemical reactions, detergents and more importantly in the organization living organisms. Hydrophilic parts of chemicals often carry an electric charge or a polar moiety, and they are attracted to each other by charge-charge or dipole-dipole interactions. Hydrophobic parts of molecules are usually alkanes or aromatic in nature, and they are attracted to each other mostly by van der Waals forces.
Solubility
The relative hydrophilicity or hydrophicity of chemical compounds plays are large role in determining which solvents a particular chemical will be soluble in, and thus determines the conditions needed to perform a chemical reaction. A reaction between two hydrophilic compounds is very unlikely to occur in benzene, for example, because it is very hydrophobic.
Soaps
Soaps and detergents[1] owe their action to a combination of hydrophilic and hydrophobic ends on the same molecule. The hydrophic ends are attracted to each other, and grease, for example, by hydrophobic interactions. The hydrophilic ends of soap molecules are attracted to water. The result is that the hydrophobic aliphatic ends of the soap molecules surround the dirt or grease, with the hydrophilic ends pointing outwards, thus making the grease removable as a spherical micelle. The grease is said to be emulsified, that is, held in solution in a solvent in which it is not normally soluble.
Cells
All biological cells maintain micelle bilayers that separate the cell from the outside world. These bilayers are formed when the hydrophilic ends of long fatty acids are attacked to each other, leaving the hydrophobic ends displayed to the outside world. Polar chemicals then need to be escorted into and out of the cell by transporter proteins or specialized pores to get through this hydrophobic region.
References
- ↑ Soaps are sodium salts of fatty acids, which tend to precipitate in hard water containing magnesium or calcium ions, leaving a scum. Detergents contain other hydrophlic groups, like sulfate, to reduce scum buildup.