Myxoma virus: Difference between revisions
imported>Lawrence Connolly mNo edit summary |
mNo edit summary |
||
| (148 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | |||
{{ | {{Taxobox | ||
| color=violet | |||
| name = Myxoma Virus | |||
| image = myxoma_virus.jpg | |||
| virus_group = Group I dsDNA virus, no RNA stage | |||
| familia = Poxviridae, subfamily: Chordopoxvirinae | |||
| genus = Leporipoxvirus}} | |||
== Classification: == | |||
''' | |||
ICTVdB Virus Code: 00.058.1.05.001. Virus accession number: 58105001. Obsolete virus code: 58.1.5.0.001; superseded accession number: 58150001. NCBI Taxon Identifier NCBI Taxonomy ID: 10273. Type of the genus: 00.058.1.05. [http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.058.1.05.htm]poripoxvirus]|Leporipoxvirus subfamily 00.058.1. [Chordopoxvirinae]|[http://www.ncbi.nlm.nih.gov/ICTVdb/Ictv/index.htm] in the family 00.058. Poxviridae.] | |||
Viruses: Group I dsDNA viruses, no RNA stage; Family: Poxviridae; SubFamily: Chordopoxvirinae; Genus: Leporipoxvirus | |||
''' | '''[[Image:Leporipoxvirus edited.jpg]] | ||
== Description and significance: == | |||
''' | |||
Myxoma virus is a member of the [[Poxviridae]] family. It causes a benign infection in [[rabbits]] of the [[Sylvilagus]] [[genus]], but induces a fatal disease known as [[myxomatosis]] in the European rabbit, [[Oryctalagus cuniculus]]. <ref>Stanford, MM, Barrett, JW, Nazarian, SH, Werden, S and McFadden, G. (2007). Oncolytic virotherapy synergism with signaling inhibitors: Rapamycin increases myxoma virus tropism for human tumor cells. Journal of Virology, 81: 1251–1260.</ref> | |||
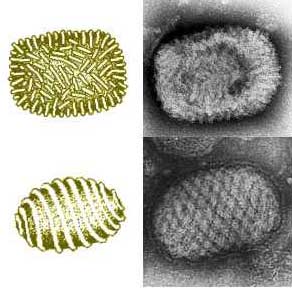
Myxoma [[virions]] have two types of structures, either enveloped or not, a surface membrane, a core, and lateral bodies. The envelope contains [[lipids]] derived from the host and [[glycolipids]] that are self synthesized. Over the course of its life cycle, myxoma virions produce both extracellular and intracellular particles. They can have two [[phenotypes]] and they can be enveloped during their extracellular phase. The extracellular virions are the ones to initiate viral infection. Myxoma virions may be segregated within [[inclusion bodies]]. They typically contain one enveloped [[nucleocapsid]], are somewhat [[pleiomorphic]], brick–shaped, and measure approximately 250 nm in diameter, 250–300 nm in length, and 200 nm in height. The core is biconcave with two lateral bodies. It lies either between the core membrane or the surface membrane. Myxoma virions mature by budding through the membrane of the host cell.<ref>Stanford, MM, Werden, SJ and McFadden, G. (2007). Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet Res 38: 299–318</ref> | |||
The Myxoma virus was important enough to have its genome sequenced is because it encodes [[proteins]] designed to circumvent the host's cellular [[immune response]] to the viral infection. This induces extensive [[immunosuppression]] in infected rabbits.<ref>Stanford, MM, and McFadden, G. (2007) Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opinion on Biological Therapy, Vol. 7, No. 9, Pages 1415-1425.</ref> | |||
''' | ''' | ||
== Natural Host: == | |||
''' | |||
Domain Eucarya, Kingdom Animalia, Phylum Chordata, Subphylum Vertebrata, Class Mammalia, Order Lagomorphia, purportedly only in Oryctolagus cuniculus, Lepus Europaeus, S. Bachmani, and S. floridanus. | |||
'''When was your organism discovered | ''' | ||
== When was your organism discovered? == | |||
''' | |||
Myxoma virus was first discovered when it killed imported European rabbits in Giuseppe Sanarelli's lab in Uruguay in 1896 at the Institute of Hygiene in Montivideo. <ref>Stanford, MM, Werden, SJ and McFadden, G. (2007). Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet Res 38: 299–318</ref> | |||
''' | ''' | ||
== How and where it was isolated: == | |||
''' | |||
The Lausanne strain of the virus was isolated by a team of Canadian scientists at the Department of Microbiology, The University of Western Ontario, London, Ontario, Canada. However, only a partial sequencing of the California MSW strain was achieved by a team associated with School of Biochemistry and Molecular Biology, Faculty of Science, Australian National University, Canberra, Australia. There they cloned EcoRI and SalI restriction fragments of viral DNA and sequenced the ends.<ref>Labudovic, A., Perkins, H. van Leeuwen, B. and Kerr, P. (2004) Sequence mapping of the Californian MSW strain of Myxoma virus. Archives of Virology, Vol. 149, Number 3/March, 553-570.</ref> | |||
''' | |||
''' | == Genome structure: == | ||
''' | |||
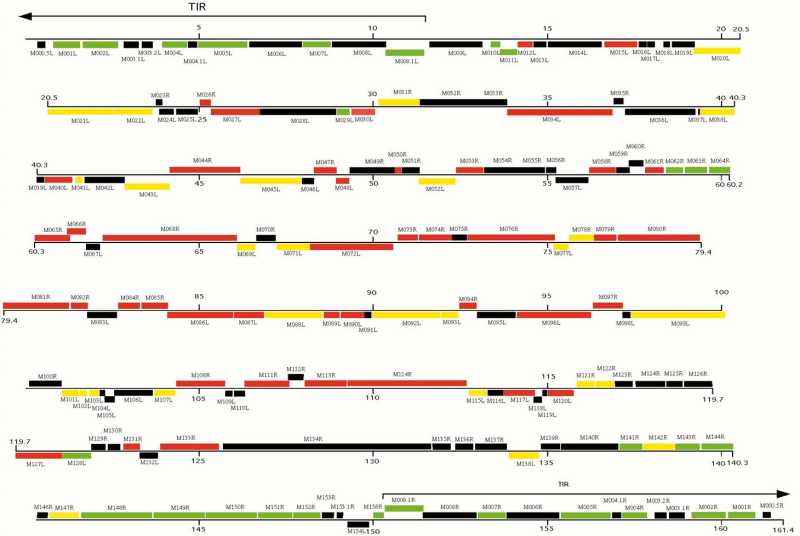
The [[genome]] is not segmented and consists of a single molecule of linear double-stranded DNA, ie. [[dsDNA]]. Sequence has the [[accession number]] [M93049]. The genome is 161,773 [[nucleotides]] long and has a central region of highly conserved enzymatic and structural genes that control essential viral functions. At both ends however, are terminal sequences with cross-linked single-stranded loops which form one continuous polynucleotide chain. These sequences include two copies of 12 genes which encode nonessential factors that affect the host's response to infection. These factors include [[serine proteinase]] inhibitors, such as SERP1, Serp2, and Serp3, and a scrapin. They are responsible for major [[histo-compatibility]] complex class I down regulation. Additionally, the genome has a guanine + cytosine content of approximately 40%. <ref>Cameron, C, Hota-Mitchell, S, Chen, L, Barrett, J, Cao, JX, Macaulay, C et al. (1999). The Complete DNA Sequence of Myxoma Virus. Virology 264: 298–318.</ref> | |||
''' | ''' | ||
[[Image:Myxoma Genome.jpg]] | |||
== Interesting Features: == | |||
' | ''' | ||
Myxoma virus subverts the host immune response using two distinct viral mechanisms, each delivered by viral proteins. Most significantly, the virus produces encoded proteins known as [[viroceptors]] or [[virokines]] which mimic host receptors or [[cytokines]]. These viroceptors or virokines act to block extracellular immune signals, thereby providing effective clearance and producing a "virus friendly" environment. Secondly, the virus uses intracellular viral proteins to impede innate antiviral responses such as [[apoptosis]], and to thwart an infected cell's mechanisms to communicate with its immune system. Additionally, the [[M128L]] myxoma virus gene expresses a five-membrane spanning cell surface protein that has amino acid homology to cellular [[CD47]] proteins. CD47 proteins are associated with determining leukocyte adhesion, motility, activation, and phagocytosis. M128L is necessary for the production of a lethal infection in rabbits. However it is not essential for the dissemination of virus within the host. The M128L protein is a novel CD47-like immunomodulatory gene of myxoma virus required for full [[pathogenesis]] of the virus. Without it, [[monocyte]] /[[macrophage]] activation is increased during infection.<ref>Iannello,A., Debbeche,O., Martin, E., Habiba Attalah, L., Samarani, S. and Ahmad, A., Viral strategies for evading antiviral cellular immune responses of the host. J. Leukoc. Biol. 2006 79: 16-35. </ref> <ref>Cameron, C. M., Barrett, J. W., Mann, M., Lucas, A., McFadden, G.. Myxoma virus M128L is expressed as a cell surface CD47-like virulence factor that contributes to the downregulation of macrophage activation in vivo. Virology , 2005 (Vol. 337)(No. 1) 55-67</ref> | |||
''' | |||
== How does this organism cause disease? == | |||
''' | |||
Myxoma virus (MV) is a [[poxvirus]] and a prototypical member of the [[Leporipoxvirus]] genus. It is the causative agent of [[myxomatosis]], a lethal and severely deblilitating disease of European rabbits (Oryctolagus cuniculus). The disease is characterized by systemic cellular [[immunosuppression]] which prompts respiratory complications and death. The myxoma virus encodes multiple proteins capable of [[downregulating]] the host innate and acquired immune responses. Other virus-encoded proteins enable replication in host [[lymphocytes]] and [[monocytes]] by inhibiting apoptosis. Specifically, Myxoma virus prevents [[apoptosis]] in [[RK-13]] cells and forms thick dermal lesions. MV encodes the virulence factor SERP2, a serine proteinase inhibitor. Virulence may depend on inhibition of pro-inflammatory [[proteinases]] by SERP2. | |||
However, notwithstanding the increasingly detailed molecular knowledge of myxoma virus, relatively little is known about the dynamics of the interaction of the virus with the integrated host-immune system during infection.<ref>MacNeill AL, Turner PC, Moyer RW, (2006) Mutation of the Myxoma virus SERP2 P1-site to prevent proteinase inhibition causes apoptosis in cultured RK-13 cells and attenuates disease in rabbits, but mutation to alter specificity causes apoptosis without reducing virulence. Virology. 2006 Dec 5-20;356(1-2):12-22. Epub 2006 Sep 7</ref><ref>Kerr P, McFadden G. (2002) Immune responses to myxoma virus. Viral Immunol. 15(2):229-46.</ref> | |||
''' | |||
== What makes it biologically interesting? == | |||
''' | |||
'''Its application to Biotechnology... its medical importance... major research findings made with it... what's cool about myxoma virus as an organism:''' | |||
*Myxoma virus has | *Myxoma virus has potential as an [[oncolytic]] [[virotherapeutic]] agent against human malignant [[glioma]] because of: 1) the nonpathogenic nature of myxoma virus outside of its host, 2) its capacity to be genetically modified, 3) its ability to produce a long-lived infection in human tumor cells, and 4) the lack of preexisting antibodies in the human population.<ref>Yang, WQ, Lun, X, Palmer, CA, Wilcox, ME, Muzik, H, Shi, ZQ, Dyck, R, Coffey, M, Thompson, B, Hamilton, M, Nishikawa, S, Brasher, P, Fonseca, K, George, D, Rewcastle, NB, Johnston, R, Stewart, D, Lee, P, Senger, D, Forsyth, P, (2004) Efficacy and Safety Evaluation of Human Reovirus Type 3 in Immunocompetent Animals, Clinical Cancer Research Vol. 10, 8561-8576</ref><ref>Lun, X, Yang, W, Alain, T, Shi, ZQ, Muzik, H, Barrett, JW et al.(2005). Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res 65: 9982–9990.</ref> | ||
*Myxoma virus selectively infects and kills human tumor cells. This capability is linked to dysregulated intracellular signalling pathways found in the majority of human cancers.<ref>Lun, XQ, Zhou, H, Alain, T, Sun, B, Wang, L, Barrett, JW et al.(2007). Targeting human [[medulloblastoma]]: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res 67: 8818–8827.</ref> | |||
*Myxoma virus appears to be an effective oncolytic agent against medulloblastoma. Whether used alone or in combination with rapamycin, myxoma virus was found to be effective and safe when used in experimental models of medulloblastoma in vitro and in vivo. Nine out of 10 medulloblastoma cell lines tested were susceptible to lethal myxoma virus infection. Additionally, it was found that the oncolytic potential myxoma virus was enhanced by combination therapy with [[signaling inhibitors]] that modulate activity of the [[phosphatidylinositol 3-kinase/Akt pathway]]. Apparently, the susceptibility of human cancer cells to be infected and killed by an oncolytic poxvirus, myxoma virus (MV), is related to the basal level of endogenous phosphorylated Akt.<ref>Lun, XQ, Zhou, H, Alain, T, Sun, B, Wang, L, Barrett, JW et al.(2007). Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res 67: 8818–8827.</ref> | |||
*All [[rhabdoid]] tumor cell lines tested in vitro were found to be susceptible to lethal infections by myxoma virus. Intraturmoral injection of live MV substantially reduced the size of subcutaneous rhabdoid tumor xenografts compared with control animals.<ref>Wu Y, Lun X, Zhou H, Wang L, Sun B, Bell JC, Barrett JW, McFadden G, Biegel JA, Senger DL, Forsyth PA.Authors' Affiliations: Departments of Oncology, Clinical Neurosciences, and Biochemistry and Molecular Biology, University of Calgary, the Tom Baker Cancer Centre, and the Clark H. Smith Brain Tumour Research Centre, Calgary, Alberta, Canada. Clinical Cancer Research, 2008 Feb 15;14(4):1218-27.</ref> | |||
Current Research | *Myxoma virus has been used successfully to treat human glioma xenografts in [[immunodeficient]] mice. Several mouse tumor cell lines, including B16 [[melanomas]], are permissive of MV infection. Multiple intratumoral injections of MV resulted in substantial tumor growth inhibition. Moreover, with systemic injection of MV in a lung [[metastasis]] model with [[B16F10LacZ]] cells substantially reduced lung tumors.<ref>Stanford, MM, Shaban, M, Barrett, J, Werden, SJ, Gilbert, PA, Bondy-Denomy, J, MacKenzie, L, Graham, K, Chambers, F, and McFadden, G, Myxoma Virus Oncolysis of Primary and Metastatic B16F10 Mouse Tumors In Vivo. Molecular Therapy (2007) 16 1, 52–59.</ref> | ||
References [ | ''' | ||
== Current Research: == | |||
''' | |||
'''Myxoma Virus Oncolysis of Primary and Metastatic B16F10 Mouse Tumors In Vivo'''<ref>Stanford, MM, Shaban, M, Barrett, J, Werden, SJ, Gilbert, PA, Bondy-Denomy, J, MacKenzie, L, Graham, K, Chambers, F, and McFadden, G, Myxoma Virus Oncolysis of Primary and Metastatic B16F10 Mouse Tumors In Vivo. Molecular Therapy (2007) 16 1, 52–59.</ref> | |||
This paper investigates the effectiveness of myxoma virus (MV) in treating primary and metastatic mouse tumors in immunocompetent C57BL6 mice. The authors found several mouse tumor cell lines, including B16 melanomas, to be permissive to MV infection. They used B16F10 cells to assess MV replication and efficacy in genetically similar primary tumor and metastatic models in vivo. Multiple intratumoral injections of MV caused substantial inhibition of tumor growth. Moreover, systemic administration of MV in a lung metastasis model with B16F10LacZ cells dramatically reduced lung tumors. Of particular note, a combination therapy of MV with rapamycin reduced both the size and number of lung metastases, as well as the induced antiviral neutralizing antibody titres. This study demonstrates that MV is capable of targeting and destroying tumors while causing no significant disease or collateral tissue infection in an immunocompetent host. Moreover when MV is combined rapamycin, the potential of MV is significant in oncolytic cancer therapy. | |||
'''Oncolytic efficacy of recombinant vesicular stomatitis virus and myxoma virus in experimental models of rhabdoid tumors.'''<ref>Wu Y, Lun X, Zhou H, Wang L, Sun B, Bell JC, Barrett JW, McFadden G, Biegel JA, Senger DL, Forsyth PA.Authors' Affiliations: Departments of Oncology, Clinical Neurosciences, and Biochemistry and Molecular Biology, University of Calgary, the Tom Baker Cancer Centre, and the Clark H. Smith Brain Tumour Research Centre, Calgary, Alberta, Canada. Clinical Cancer Research, 2008 Feb 15;14(4):1218-27.</ref> | |||
This paper investigates the therapeutic potential of two oncolytic viruses, myxoma virus (MV) and an attenuated vesicular stomatitis virus (VSV(DeltaM51)) in models of human rhabdoid tumor. Rhabdoid tumors are highly aggressive pediatric tumors that are typically unsusceptible to treatment. In this experiment, four human rhabdoid tumor cell lines were cultured in vitro and treated with live or inactivated control virus. At various times after infection, the cytopathic effect, the viral gene expression, the infectious viral titers, and cell viability were measured. However, to gain insight into viral oncolysis in vivo, human rhabdoid tumor cells were implanted subcutaneously or intracranially in CD-1 nude mice, which were then treated with intratumoral or i.v. injections of live or UV-inactivated virus. | |||
In terms of results: 1) all in vitro rhabdoid tumor cell lines were susceptible to lethal infections by MV and VSV(DeltaM51); and 2)Intratumoral injection of live MV or VSV(DeltaM51) reduced the size of s.c. rhabdoid tumor xenografts "dramatically" when compared with control animals. Consequently, these results indicate that VSV(DeltaM51) and MV have potential as novel therapies against human rhabdoid tumor. | |||
'''Oncolytic Virotherapy Synergism with Signaling Inhibitors: Rapamycin Increases Myxoma Virus Tropism for Human Tumor Cells'''<ref>Marianne M. Stanford, John W. Barrett, Steven H. Nazarian, Steven Werden, and Grant McFadden* Biotherapeutics Research Group, Robarts Research Institute, and Department of Microbiology and Immunology, University of Western Ontario, London, Ontario N6G 2V4, Canada Journal of Virology, 2007 February; 81(3): 1251–1260.</ref> | |||
This paper investigates the effect of treating non-permissive human tumor cell lines, which usually restrict myxoma virus replication, with rapamycin. Aside from being a rabbit-specific poxvirus pathogen, myxoma virus also has a unique tropism for human tumor cells and is substantially oncolytic for human cancer xenografts. Apparently most tumor cell lines are permissive for myxoma infection as a consequence of activation of Akt kinase. M-T5, a range factor of myxoma virus, directly interacts with Akt and mediates myxoma virus tumor cell tropism. Rapamycin specifically inhibits mTOR, a regulator of cell growth and metabolism downstream of Akt. Non-permissive human tumor cell lines were treated with rapamycin. The result was a dramatic increase in virus tropism and transmission in vitro. This increased myxoma replication occurred correspondingly with the effects on mTOR signaling, specifically, an increase in Akt kinase. However, in contrast, rapamycin does not increase myxoma virus replication in rabbit cell lines or permissive human tumor cell lines with active Akt. This finding is significant in that it indicates that rapamycin increases the oncolytic capacity of myxoma virus for human cancer cells by reconfiguring the internal cell signaling environment to be optimal for virus replication. It also suggests that a potentially therapeutic synergy exists between kinase signaling inhibitors and oncolytic poxviruses for cancer treatment. | |||
''' | |||
'''Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin.'''<ref>Lun XQ, Zhou H, Alain T, Sun B, Wang L, Barrett JW, Stanford MM, McFadden G, Bell J, Senger DL, Forsyth PA. Department of Oncology, Tom Baker Cancer Centre, University of Calgary, Calgary, Alberta, Canada. Cancer Research, 2007 Sep 15;67(18):8818-27.</ref> | |||
This paper investigates and demonstrates that myxoma virus either used alone or in combination with rapamycin is effective and safe experimental models of medulloblastoma in vitro and in vivo. Nine out of 10 medulloblastoma cell lines tested were found to be susceptible to lethal myxoma virus infection. However, pretreatment of medulloblastoma cells with rapamycin increased the extent of oncolysis in vitro. In terms of experimental protocol, intratumoral injection of live myxoma virus prolonged survival in D341 and Daoy orthotopic human medulloblastoma xenograft mouse models as compared to the inactivated virus control. Pretreatment with rapamycin increased the extent of viral oncolysis, effectively "curing" most Daoy tumor-bearing mice and reducing or eliminating spinal cord and ventricle metastases. Moreover, rapamycin enhanced tumor-specific myxoma virus replication in vivo and prolonged survival of D341 tumor-bearing mice. Significantly, rapamycin increased the levels of activated Akt in Daoy and D341 cells, a finding that susgests an explanation for its ability to enhance myxoma virus oncolysis. In sum, these findings suggest: 1) that myxoma virus may be an effective oncolytic agent against medulloblastoma, and 2) that therapy with signaling inhibitors that affect the phosphatidylinositol 3-kinase/Akt pathway will further enhance the oncolytic potential of myxoma virus. | |||
== References: == | |||
<references/> | |||
''' | |||
*Barrett, JW, Sypula, J, Wang, F, Alston, LR, Shao, Z, Gao, X et al.(2007). M135R is a novel cell surface virulence factor of myxoma virus. J Virol 81: 106–114. | |||
*Cameron, C, Hota-Mitchell, S, Chen, L, Barrett, J, Cao, JX, Macaulay, C et al. (1999). The Complete DNA Sequence of Myxoma Virus. Virology 264: 298–318. | |||
*Cameron, C. M., Barrett, J. W., Mann, M., Lucas, A., McFadden, G.. Myxoma virus M128L is expressed as a cell surface CD47-like virulence factor that contributes to the downregulation of macrophage activation in vivo. Virology, 2005 (Vol. 337)(No. 1) 55-67 | |||
*Duteyrata, J. et al., (2001) Ultrastructural morphogenesis study of myxoma virus replication cycle. Biology of the Cell 93 349–361. | |||
*Fenner, F. (2000). Adventures with poxviruses of vertebrates. FEMS Microbiol Rev 24: 123–133. | |||
*Iannello,A., Debbeche,O., Martin, E., Habiba Attalah, L., Samarani, S. and Ahmad, A., Viral strategies for evading antiviral cellular immune responses of the host. J. Leukoc. Biol. 2006 79: 16-35. | |||
*Kerr P, McFadden G. (2002) Immune responses to myxoma virus. Viral Immunol. 15(2):229-46. | |||
*Labudovic, A., Perkins, H. van Leeuwen, B. and Kerr, P. (2004) Sequence mapping of the Californian MSW strain of Myxoma virus. Archives of Virology, Vol. 149, Number 3/March, 553-570. | |||
*Lun,X, Yang,W, Alain,T, Shi,ZQ, Muzik,H, Barrett,JW et al.(2005). Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res 65: 9982–9990. | |||
*Lun, XQ, Zhou, H, Alain, T, Sun, B, Wang, L, Barrett, JW et al.(2007). Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res 67: 8818–8827. | |||
*MacNeill AL, Turner PC, Moyer RW, (2006) Mutation of the Myxoma virus SERP2 P1-site to prevent proteinase inhibition causes apoptosis in cultured RK-13 cells and attenuates disease in rabbits, but mutation to alter specificity causes apoptosis without reducing virulence. Virology. 2006 Dec 5-20;356(1-2):12-22. Epub 2006 Sep 7. | |||
*Stanford, MM, Shaban, M, Barrett, J, Werden, SJ, Gilbert, PA, Bondy-Denomy, J, MacKenzie, L, Graham, K, Chambers, F, and McFadden, G, Myxoma Virus Oncolysis of Primary and Metastatic B16F10 Mouse Tumors In Vivo. Molecular Therapy (2007) 16 1, 52–59. | |||
*Stanford, MM, Barrett, JW, Nazarian, SH, Werden, S and McFadden, G. (2007). Oncolytic virotherapy synergism with signaling inhibitors: Rapamycin increases myxoma virus tropism for human tumor cells. Journal of Virology, 81: 1251–1260. | |||
*Stanford, MM, Werden, SJ and McFadden, G. (2007). Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet Res 38: 299–318 | |||
*Stanford, MM, and McFadden, G. (2007) Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opinion on Biological Therapy, Vol. 7, No. 9, Pages 1415-1425. | |||
*Sypula, J, Wang, F, Ma, Y, Bell, JC and McFadden, G. (2004). Myxoma virus tropism in human tumor cells. Gene Ther Mol Biol 8: 108–114. | |||
*Wang, G, Barrett, JW, Stanford, M, Werden, SJ, Johnston, JB, Gao, X et al.(2006). Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci USA 103: 4640–4645. | |||
*Wang G, Barrett JW, Stanford M, Werden SJ, Johnston JB, Gao X, Sun M, Cheng JQ, McFadden G. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4640-5. Epub 2006 Mar 14. | |||
*Yang, WQ, Lun, X, Palmer, CA, Wilcox, ME, Muzik, H, Shi, ZQ, Dyck, R, Coffey, M, Thompson, B, Hamilton, M, Nishikawa, S, Brasher, P, Fonseca, K, George, D, Rewcastle, NB, Johnston, R, Stewart, D, Lee, P, Senger, D, Forsyth, P, (2004) Efficacy and Safety Evaluation of Human Reovirus Type 3 in Immunocompetent Animals, Clinical Cancer Research Vol. 10, 8561-8576[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:00, 22 September 2024
| Myxoma Virus | ||||||
|---|---|---|---|---|---|---|
 | ||||||
| Virus classification | ||||||
|
Classification:
ICTVdB Virus Code: 00.058.1.05.001. Virus accession number: 58105001. Obsolete virus code: 58.1.5.0.001; superseded accession number: 58150001. NCBI Taxon Identifier NCBI Taxonomy ID: 10273. Type of the genus: 00.058.1.05. [1]poripoxvirus]|Leporipoxvirus subfamily 00.058.1. [Chordopoxvirinae]|[2] in the family 00.058. Poxviridae.]
Viruses: Group I dsDNA viruses, no RNA stage; Family: Poxviridae; SubFamily: Chordopoxvirinae; Genus: Leporipoxvirus
Description and significance:
Myxoma virus is a member of the Poxviridae family. It causes a benign infection in rabbits of the Sylvilagus genus, but induces a fatal disease known as myxomatosis in the European rabbit, Oryctalagus cuniculus. [1] Myxoma virions have two types of structures, either enveloped or not, a surface membrane, a core, and lateral bodies. The envelope contains lipids derived from the host and glycolipids that are self synthesized. Over the course of its life cycle, myxoma virions produce both extracellular and intracellular particles. They can have two phenotypes and they can be enveloped during their extracellular phase. The extracellular virions are the ones to initiate viral infection. Myxoma virions may be segregated within inclusion bodies. They typically contain one enveloped nucleocapsid, are somewhat pleiomorphic, brick–shaped, and measure approximately 250 nm in diameter, 250–300 nm in length, and 200 nm in height. The core is biconcave with two lateral bodies. It lies either between the core membrane or the surface membrane. Myxoma virions mature by budding through the membrane of the host cell.[2] The Myxoma virus was important enough to have its genome sequenced is because it encodes proteins designed to circumvent the host's cellular immune response to the viral infection. This induces extensive immunosuppression in infected rabbits.[3]
Natural Host:
Domain Eucarya, Kingdom Animalia, Phylum Chordata, Subphylum Vertebrata, Class Mammalia, Order Lagomorphia, purportedly only in Oryctolagus cuniculus, Lepus Europaeus, S. Bachmani, and S. floridanus.
When was your organism discovered?
Myxoma virus was first discovered when it killed imported European rabbits in Giuseppe Sanarelli's lab in Uruguay in 1896 at the Institute of Hygiene in Montivideo. [4]
How and where it was isolated:
The Lausanne strain of the virus was isolated by a team of Canadian scientists at the Department of Microbiology, The University of Western Ontario, London, Ontario, Canada. However, only a partial sequencing of the California MSW strain was achieved by a team associated with School of Biochemistry and Molecular Biology, Faculty of Science, Australian National University, Canberra, Australia. There they cloned EcoRI and SalI restriction fragments of viral DNA and sequenced the ends.[5]
Genome structure:
The genome is not segmented and consists of a single molecule of linear double-stranded DNA, ie. dsDNA. Sequence has the accession number [M93049]. The genome is 161,773 nucleotides long and has a central region of highly conserved enzymatic and structural genes that control essential viral functions. At both ends however, are terminal sequences with cross-linked single-stranded loops which form one continuous polynucleotide chain. These sequences include two copies of 12 genes which encode nonessential factors that affect the host's response to infection. These factors include serine proteinase inhibitors, such as SERP1, Serp2, and Serp3, and a scrapin. They are responsible for major histo-compatibility complex class I down regulation. Additionally, the genome has a guanine + cytosine content of approximately 40%. [6]
Interesting Features:
Myxoma virus subverts the host immune response using two distinct viral mechanisms, each delivered by viral proteins. Most significantly, the virus produces encoded proteins known as viroceptors or virokines which mimic host receptors or cytokines. These viroceptors or virokines act to block extracellular immune signals, thereby providing effective clearance and producing a "virus friendly" environment. Secondly, the virus uses intracellular viral proteins to impede innate antiviral responses such as apoptosis, and to thwart an infected cell's mechanisms to communicate with its immune system. Additionally, the M128L myxoma virus gene expresses a five-membrane spanning cell surface protein that has amino acid homology to cellular CD47 proteins. CD47 proteins are associated with determining leukocyte adhesion, motility, activation, and phagocytosis. M128L is necessary for the production of a lethal infection in rabbits. However it is not essential for the dissemination of virus within the host. The M128L protein is a novel CD47-like immunomodulatory gene of myxoma virus required for full pathogenesis of the virus. Without it, monocyte /macrophage activation is increased during infection.[7] [8]
How does this organism cause disease?
Myxoma virus (MV) is a poxvirus and a prototypical member of the Leporipoxvirus genus. It is the causative agent of myxomatosis, a lethal and severely deblilitating disease of European rabbits (Oryctolagus cuniculus). The disease is characterized by systemic cellular immunosuppression which prompts respiratory complications and death. The myxoma virus encodes multiple proteins capable of downregulating the host innate and acquired immune responses. Other virus-encoded proteins enable replication in host lymphocytes and monocytes by inhibiting apoptosis. Specifically, Myxoma virus prevents apoptosis in RK-13 cells and forms thick dermal lesions. MV encodes the virulence factor SERP2, a serine proteinase inhibitor. Virulence may depend on inhibition of pro-inflammatory proteinases by SERP2. However, notwithstanding the increasingly detailed molecular knowledge of myxoma virus, relatively little is known about the dynamics of the interaction of the virus with the integrated host-immune system during infection.[9][10]
What makes it biologically interesting?
Its application to Biotechnology... its medical importance... major research findings made with it... what's cool about myxoma virus as an organism:
- Myxoma virus has potential as an oncolytic virotherapeutic agent against human malignant glioma because of: 1) the nonpathogenic nature of myxoma virus outside of its host, 2) its capacity to be genetically modified, 3) its ability to produce a long-lived infection in human tumor cells, and 4) the lack of preexisting antibodies in the human population.[11][12]
- Myxoma virus selectively infects and kills human tumor cells. This capability is linked to dysregulated intracellular signalling pathways found in the majority of human cancers.[13]
- Myxoma virus appears to be an effective oncolytic agent against medulloblastoma. Whether used alone or in combination with rapamycin, myxoma virus was found to be effective and safe when used in experimental models of medulloblastoma in vitro and in vivo. Nine out of 10 medulloblastoma cell lines tested were susceptible to lethal myxoma virus infection. Additionally, it was found that the oncolytic potential myxoma virus was enhanced by combination therapy with signaling inhibitors that modulate activity of the phosphatidylinositol 3-kinase/Akt pathway. Apparently, the susceptibility of human cancer cells to be infected and killed by an oncolytic poxvirus, myxoma virus (MV), is related to the basal level of endogenous phosphorylated Akt.[14]
- All rhabdoid tumor cell lines tested in vitro were found to be susceptible to lethal infections by myxoma virus. Intraturmoral injection of live MV substantially reduced the size of subcutaneous rhabdoid tumor xenografts compared with control animals.[15]
- Myxoma virus has been used successfully to treat human glioma xenografts in immunodeficient mice. Several mouse tumor cell lines, including B16 melanomas, are permissive of MV infection. Multiple intratumoral injections of MV resulted in substantial tumor growth inhibition. Moreover, with systemic injection of MV in a lung metastasis model with B16F10LacZ cells substantially reduced lung tumors.[16]
Current Research:
Myxoma Virus Oncolysis of Primary and Metastatic B16F10 Mouse Tumors In Vivo[17] This paper investigates the effectiveness of myxoma virus (MV) in treating primary and metastatic mouse tumors in immunocompetent C57BL6 mice. The authors found several mouse tumor cell lines, including B16 melanomas, to be permissive to MV infection. They used B16F10 cells to assess MV replication and efficacy in genetically similar primary tumor and metastatic models in vivo. Multiple intratumoral injections of MV caused substantial inhibition of tumor growth. Moreover, systemic administration of MV in a lung metastasis model with B16F10LacZ cells dramatically reduced lung tumors. Of particular note, a combination therapy of MV with rapamycin reduced both the size and number of lung metastases, as well as the induced antiviral neutralizing antibody titres. This study demonstrates that MV is capable of targeting and destroying tumors while causing no significant disease or collateral tissue infection in an immunocompetent host. Moreover when MV is combined rapamycin, the potential of MV is significant in oncolytic cancer therapy.
Oncolytic efficacy of recombinant vesicular stomatitis virus and myxoma virus in experimental models of rhabdoid tumors.[18] This paper investigates the therapeutic potential of two oncolytic viruses, myxoma virus (MV) and an attenuated vesicular stomatitis virus (VSV(DeltaM51)) in models of human rhabdoid tumor. Rhabdoid tumors are highly aggressive pediatric tumors that are typically unsusceptible to treatment. In this experiment, four human rhabdoid tumor cell lines were cultured in vitro and treated with live or inactivated control virus. At various times after infection, the cytopathic effect, the viral gene expression, the infectious viral titers, and cell viability were measured. However, to gain insight into viral oncolysis in vivo, human rhabdoid tumor cells were implanted subcutaneously or intracranially in CD-1 nude mice, which were then treated with intratumoral or i.v. injections of live or UV-inactivated virus. In terms of results: 1) all in vitro rhabdoid tumor cell lines were susceptible to lethal infections by MV and VSV(DeltaM51); and 2)Intratumoral injection of live MV or VSV(DeltaM51) reduced the size of s.c. rhabdoid tumor xenografts "dramatically" when compared with control animals. Consequently, these results indicate that VSV(DeltaM51) and MV have potential as novel therapies against human rhabdoid tumor.
Oncolytic Virotherapy Synergism with Signaling Inhibitors: Rapamycin Increases Myxoma Virus Tropism for Human Tumor Cells[19] This paper investigates the effect of treating non-permissive human tumor cell lines, which usually restrict myxoma virus replication, with rapamycin. Aside from being a rabbit-specific poxvirus pathogen, myxoma virus also has a unique tropism for human tumor cells and is substantially oncolytic for human cancer xenografts. Apparently most tumor cell lines are permissive for myxoma infection as a consequence of activation of Akt kinase. M-T5, a range factor of myxoma virus, directly interacts with Akt and mediates myxoma virus tumor cell tropism. Rapamycin specifically inhibits mTOR, a regulator of cell growth and metabolism downstream of Akt. Non-permissive human tumor cell lines were treated with rapamycin. The result was a dramatic increase in virus tropism and transmission in vitro. This increased myxoma replication occurred correspondingly with the effects on mTOR signaling, specifically, an increase in Akt kinase. However, in contrast, rapamycin does not increase myxoma virus replication in rabbit cell lines or permissive human tumor cell lines with active Akt. This finding is significant in that it indicates that rapamycin increases the oncolytic capacity of myxoma virus for human cancer cells by reconfiguring the internal cell signaling environment to be optimal for virus replication. It also suggests that a potentially therapeutic synergy exists between kinase signaling inhibitors and oncolytic poxviruses for cancer treatment.
Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin.[20] This paper investigates and demonstrates that myxoma virus either used alone or in combination with rapamycin is effective and safe experimental models of medulloblastoma in vitro and in vivo. Nine out of 10 medulloblastoma cell lines tested were found to be susceptible to lethal myxoma virus infection. However, pretreatment of medulloblastoma cells with rapamycin increased the extent of oncolysis in vitro. In terms of experimental protocol, intratumoral injection of live myxoma virus prolonged survival in D341 and Daoy orthotopic human medulloblastoma xenograft mouse models as compared to the inactivated virus control. Pretreatment with rapamycin increased the extent of viral oncolysis, effectively "curing" most Daoy tumor-bearing mice and reducing or eliminating spinal cord and ventricle metastases. Moreover, rapamycin enhanced tumor-specific myxoma virus replication in vivo and prolonged survival of D341 tumor-bearing mice. Significantly, rapamycin increased the levels of activated Akt in Daoy and D341 cells, a finding that susgests an explanation for its ability to enhance myxoma virus oncolysis. In sum, these findings suggest: 1) that myxoma virus may be an effective oncolytic agent against medulloblastoma, and 2) that therapy with signaling inhibitors that affect the phosphatidylinositol 3-kinase/Akt pathway will further enhance the oncolytic potential of myxoma virus.
References:
- ↑ Stanford, MM, Barrett, JW, Nazarian, SH, Werden, S and McFadden, G. (2007). Oncolytic virotherapy synergism with signaling inhibitors: Rapamycin increases myxoma virus tropism for human tumor cells. Journal of Virology, 81: 1251–1260.
- ↑ Stanford, MM, Werden, SJ and McFadden, G. (2007). Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet Res 38: 299–318
- ↑ Stanford, MM, and McFadden, G. (2007) Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opinion on Biological Therapy, Vol. 7, No. 9, Pages 1415-1425.
- ↑ Stanford, MM, Werden, SJ and McFadden, G. (2007). Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet Res 38: 299–318
- ↑ Labudovic, A., Perkins, H. van Leeuwen, B. and Kerr, P. (2004) Sequence mapping of the Californian MSW strain of Myxoma virus. Archives of Virology, Vol. 149, Number 3/March, 553-570.
- ↑ Cameron, C, Hota-Mitchell, S, Chen, L, Barrett, J, Cao, JX, Macaulay, C et al. (1999). The Complete DNA Sequence of Myxoma Virus. Virology 264: 298–318.
- ↑ Iannello,A., Debbeche,O., Martin, E., Habiba Attalah, L., Samarani, S. and Ahmad, A., Viral strategies for evading antiviral cellular immune responses of the host. J. Leukoc. Biol. 2006 79: 16-35.
- ↑ Cameron, C. M., Barrett, J. W., Mann, M., Lucas, A., McFadden, G.. Myxoma virus M128L is expressed as a cell surface CD47-like virulence factor that contributes to the downregulation of macrophage activation in vivo. Virology , 2005 (Vol. 337)(No. 1) 55-67
- ↑ MacNeill AL, Turner PC, Moyer RW, (2006) Mutation of the Myxoma virus SERP2 P1-site to prevent proteinase inhibition causes apoptosis in cultured RK-13 cells and attenuates disease in rabbits, but mutation to alter specificity causes apoptosis without reducing virulence. Virology. 2006 Dec 5-20;356(1-2):12-22. Epub 2006 Sep 7
- ↑ Kerr P, McFadden G. (2002) Immune responses to myxoma virus. Viral Immunol. 15(2):229-46.
- ↑ Yang, WQ, Lun, X, Palmer, CA, Wilcox, ME, Muzik, H, Shi, ZQ, Dyck, R, Coffey, M, Thompson, B, Hamilton, M, Nishikawa, S, Brasher, P, Fonseca, K, George, D, Rewcastle, NB, Johnston, R, Stewart, D, Lee, P, Senger, D, Forsyth, P, (2004) Efficacy and Safety Evaluation of Human Reovirus Type 3 in Immunocompetent Animals, Clinical Cancer Research Vol. 10, 8561-8576
- ↑ Lun, X, Yang, W, Alain, T, Shi, ZQ, Muzik, H, Barrett, JW et al.(2005). Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res 65: 9982–9990.
- ↑ Lun, XQ, Zhou, H, Alain, T, Sun, B, Wang, L, Barrett, JW et al.(2007). Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res 67: 8818–8827.
- ↑ Lun, XQ, Zhou, H, Alain, T, Sun, B, Wang, L, Barrett, JW et al.(2007). Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res 67: 8818–8827.
- ↑ Wu Y, Lun X, Zhou H, Wang L, Sun B, Bell JC, Barrett JW, McFadden G, Biegel JA, Senger DL, Forsyth PA.Authors' Affiliations: Departments of Oncology, Clinical Neurosciences, and Biochemistry and Molecular Biology, University of Calgary, the Tom Baker Cancer Centre, and the Clark H. Smith Brain Tumour Research Centre, Calgary, Alberta, Canada. Clinical Cancer Research, 2008 Feb 15;14(4):1218-27.
- ↑ Stanford, MM, Shaban, M, Barrett, J, Werden, SJ, Gilbert, PA, Bondy-Denomy, J, MacKenzie, L, Graham, K, Chambers, F, and McFadden, G, Myxoma Virus Oncolysis of Primary and Metastatic B16F10 Mouse Tumors In Vivo. Molecular Therapy (2007) 16 1, 52–59.
- ↑ Stanford, MM, Shaban, M, Barrett, J, Werden, SJ, Gilbert, PA, Bondy-Denomy, J, MacKenzie, L, Graham, K, Chambers, F, and McFadden, G, Myxoma Virus Oncolysis of Primary and Metastatic B16F10 Mouse Tumors In Vivo. Molecular Therapy (2007) 16 1, 52–59.
- ↑ Wu Y, Lun X, Zhou H, Wang L, Sun B, Bell JC, Barrett JW, McFadden G, Biegel JA, Senger DL, Forsyth PA.Authors' Affiliations: Departments of Oncology, Clinical Neurosciences, and Biochemistry and Molecular Biology, University of Calgary, the Tom Baker Cancer Centre, and the Clark H. Smith Brain Tumour Research Centre, Calgary, Alberta, Canada. Clinical Cancer Research, 2008 Feb 15;14(4):1218-27.
- ↑ Marianne M. Stanford, John W. Barrett, Steven H. Nazarian, Steven Werden, and Grant McFadden* Biotherapeutics Research Group, Robarts Research Institute, and Department of Microbiology and Immunology, University of Western Ontario, London, Ontario N6G 2V4, Canada Journal of Virology, 2007 February; 81(3): 1251–1260.
- ↑ Lun XQ, Zhou H, Alain T, Sun B, Wang L, Barrett JW, Stanford MM, McFadden G, Bell J, Senger DL, Forsyth PA. Department of Oncology, Tom Baker Cancer Centre, University of Calgary, Calgary, Alberta, Canada. Cancer Research, 2007 Sep 15;67(18):8818-27.
- Barrett, JW, Sypula, J, Wang, F, Alston, LR, Shao, Z, Gao, X et al.(2007). M135R is a novel cell surface virulence factor of myxoma virus. J Virol 81: 106–114.
- Cameron, C, Hota-Mitchell, S, Chen, L, Barrett, J, Cao, JX, Macaulay, C et al. (1999). The Complete DNA Sequence of Myxoma Virus. Virology 264: 298–318.
- Cameron, C. M., Barrett, J. W., Mann, M., Lucas, A., McFadden, G.. Myxoma virus M128L is expressed as a cell surface CD47-like virulence factor that contributes to the downregulation of macrophage activation in vivo. Virology, 2005 (Vol. 337)(No. 1) 55-67
- Duteyrata, J. et al., (2001) Ultrastructural morphogenesis study of myxoma virus replication cycle. Biology of the Cell 93 349–361.
- Fenner, F. (2000). Adventures with poxviruses of vertebrates. FEMS Microbiol Rev 24: 123–133.
- Iannello,A., Debbeche,O., Martin, E., Habiba Attalah, L., Samarani, S. and Ahmad, A., Viral strategies for evading antiviral cellular immune responses of the host. J. Leukoc. Biol. 2006 79: 16-35.
- Kerr P, McFadden G. (2002) Immune responses to myxoma virus. Viral Immunol. 15(2):229-46.
- Labudovic, A., Perkins, H. van Leeuwen, B. and Kerr, P. (2004) Sequence mapping of the Californian MSW strain of Myxoma virus. Archives of Virology, Vol. 149, Number 3/March, 553-570.
- Lun,X, Yang,W, Alain,T, Shi,ZQ, Muzik,H, Barrett,JW et al.(2005). Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res 65: 9982–9990.
- Lun, XQ, Zhou, H, Alain, T, Sun, B, Wang, L, Barrett, JW et al.(2007). Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res 67: 8818–8827.
- MacNeill AL, Turner PC, Moyer RW, (2006) Mutation of the Myxoma virus SERP2 P1-site to prevent proteinase inhibition causes apoptosis in cultured RK-13 cells and attenuates disease in rabbits, but mutation to alter specificity causes apoptosis without reducing virulence. Virology. 2006 Dec 5-20;356(1-2):12-22. Epub 2006 Sep 7.
- Stanford, MM, Shaban, M, Barrett, J, Werden, SJ, Gilbert, PA, Bondy-Denomy, J, MacKenzie, L, Graham, K, Chambers, F, and McFadden, G, Myxoma Virus Oncolysis of Primary and Metastatic B16F10 Mouse Tumors In Vivo. Molecular Therapy (2007) 16 1, 52–59.
- Stanford, MM, Barrett, JW, Nazarian, SH, Werden, S and McFadden, G. (2007). Oncolytic virotherapy synergism with signaling inhibitors: Rapamycin increases myxoma virus tropism for human tumor cells. Journal of Virology, 81: 1251–1260.
- Stanford, MM, Werden, SJ and McFadden, G. (2007). Myxoma virus in the European rabbit: interactions between the virus and its susceptible host. Vet Res 38: 299–318
- Stanford, MM, and McFadden, G. (2007) Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opinion on Biological Therapy, Vol. 7, No. 9, Pages 1415-1425.
- Sypula, J, Wang, F, Ma, Y, Bell, JC and McFadden, G. (2004). Myxoma virus tropism in human tumor cells. Gene Ther Mol Biol 8: 108–114.
- Wang, G, Barrett, JW, Stanford, M, Werden, SJ, Johnston, JB, Gao, X et al.(2006). Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci USA 103: 4640–4645.
- Wang G, Barrett JW, Stanford M, Werden SJ, Johnston JB, Gao X, Sun M, Cheng JQ, McFadden G. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4640-5. Epub 2006 Mar 14.
- Yang, WQ, Lun, X, Palmer, CA, Wilcox, ME, Muzik, H, Shi, ZQ, Dyck, R, Coffey, M, Thompson, B, Hamilton, M, Nishikawa, S, Brasher, P, Fonseca, K, George, D, Rewcastle, NB, Johnston, R, Stewart, D, Lee, P, Senger, D, Forsyth, P, (2004) Efficacy and Safety Evaluation of Human Reovirus Type 3 in Immunocompetent Animals, Clinical Cancer Research Vol. 10, 8561-8576