Fenske equation: Difference between revisions
imported>Milton Beychok m (→A common version of the Fenske equation: Added an internal CZ link) |
mNo edit summary |
||
| (17 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
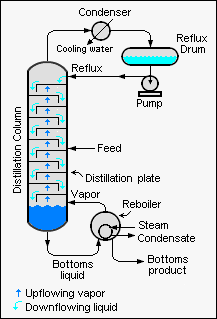
{{Image|Total Reflux.png|right|275px|Industrial distillation column operating at total reflux}} | |||
The '''Fenske equation''' is used for calculating the minimum number of [[theoretical plate]]s required for the separation of a binary feed stream by a [[ | The '''Fenske equation''' is used for calculating the minimum number of [[theoretical plate]]s required for the separation of a binary feed stream by a [[Continuous distillation|distillation column]] that is being operated at total [[Reflux (distillation)|reflux]] (i.e., which means that no overhead product is being withdrawn from the column). The derivation of the Fenske equation assumes that the [[relative volatility]] is constant in the distillation column. | ||
Theoretical plates are also often referred to as theoretical | Theoretical plates are also often referred to as [[theoretical tray]]s or [[equilibrium stage]]s. | ||
The equation was derived by Merrell | The equation was derived in 1932 by Merrell Fenske, a professor who served as the head of the [[chemical engineering]] department at the Pennsylvania State University from 1959 to 1969.<ref>{{cite journal| author=M.R. Fenske|title=Fractionation of Straight-run Pennsylvania Gasoline|journal=Industrial Engineering Chemistry|volume=24|issue=5| pages=482-485|date=May 24, 1932}}</ref> | ||
When designing large-scale, continuous industrial distillation towers, it is very useful to first calculate the minimum number of theoretical plates required to obtain the desired overhead product composition. | |||
==A common version of the Fenske equation== | ==A common version of the Fenske equation== | ||
This is one of the many different but equivalent versions of the Fenske equation:<ref name=Schreiber>[http://www.eng.fsu.edu/~schreiber/uol/exp300/ Distillation notes] (Loren Schreiber, [[Florida State University]])</ref><ref name= | This is one of the many different but equivalent versions of the Fenske equation:<ref name=Schreiber>[http://www.eng.fsu.edu/~schreiber/uol/exp300/ Distillation notes] (Loren Schreiber, [[Florida State University]])</ref><ref name=Jones>{{cite book|author=David S.J. Jones and Peter P. Pujado (Editors)|title=Handbook of Petroleum Processing|edition=First Edition|publisher=Springer|year=2006|id=ISBN 1-4020-2819-9}} [http://books.google.com/books?id=D6pb1Yn0vYoC&printsec=frontcover&dq=%22Handbook+of+Petroleum+Processing%22&lr=&as_drrb_is=q&as_minm_is=0&as_miny_is=&as_maxm_is=0&as_maxy_is=&as_brr=0#v=snippet&q=Fenske&f=false Google books] Search for "Fenske" to get to page 200.</ref><ref>[http://www.chemeng.ed.ac.uk/~jskillin/teaching/sepprocs/2004-05/tutorials/Tut6.pdf Tutorial 6: Separation Processes] (J. Skilling, [[University of Edinburgh]], Scotland)</ref><ref>{{cite book|author=Maxwell, J.B.|title=Data Book on Hydrocarbons|edition=1st Edition|publisher=D. Van Nostrand|year=1950|id=}}</ref> | ||
:<math>\ N = \frac{\log \, \bigg[ \Big(\frac{X_d}{1-X_d}\Big)\Big(\frac{1-X_b}{X_b} \Big) \bigg]}{\log \, \alpha_{avg}} </math> | :<math>\ N = \frac{\log \, \bigg[ \Big(\frac{X_d}{1-X_d}\Big)\Big(\frac{1-X_b}{X_b} \Big) \bigg]}{\log \, \alpha_{avg}} </math> | ||
| Line 31: | Line 33: | ||
|} | |} | ||
For ease of expression, the more volatile and the less volatile components are commonly referred to as the '''light key''' (LK) and the '''heavy key''' (HK), respectively. | For ease of expression, the more volatile and the less volatile components are commonly referred to as the '''light key''' (LK) and the '''heavy key''' (HK), respectively. Using that terminology, the above equation may be expressed as:<ref name=Jones/> | ||
:<math>\ N = \frac{\log \, \bigg[ \Big(\frac{LK_d}{HK_d}\Big)\Big(\frac{HK_b}{LK_b} \Big) \bigg]}{\log \, \alpha_{avg}} </math> | |||
or also: | |||
:<math>\ N = \frac{\log \, \bigg[ \Big(\frac{LK_d}{1-LK_d}\Big)\Big(\frac{1-LK_b}{LK_b} \Big) \bigg]}{\log \, \alpha_{avg}} </math> | |||
If the relative volatility of the light key to the heavy key is constant from the column top to the column bottom, then <math>\alpha_{avg.}</math> is simply <math>\alpha</math>. If the relative volatility is not constant from top to bottom of the column, then the following approximation may be used:<ref name=Schreiber/> | If the relative volatility of the light key to the heavy key is constant from the column top to the column bottom, then <math>\alpha_{avg.}</math> is simply <math>\alpha</math>. If the relative volatility is not constant from top to bottom of the column, then the following approximation may be used:<ref name=Schreiber/> | ||
| Line 49: | Line 57: | ||
|} | |} | ||
The above form of the Fenske equation can be modified for use in the total reflux distillation of multi-component feeds. | The above form of the Fenske equation can be modified for use in the total reflux distillation of multi-component feeds. | ||
==Another form of the Fenske equation== | ==Another form of the Fenske equation== | ||
Using [[Raoult's law]] and [[Dalton's law]] for a series of condensation and evaporation cycles (i.e., equilibrium stages or theoretical plates), another form of the Fenske equation is obtained for use in [[gas chromatography]]:<ref>''Separation By Distillation With Quantitative Gas Chromatography'' (08-18-09), received as a personal communication from the Chemistry Department of the U.S. Naval Academy, which is not available online.</ref> | |||
:<math>\ \frac{Z_a}{Z_b} = \frac{X_a}{X_b} \left (\frac{P^0_a}{P^0_b} \right) ^N </math> | :<math>\ \frac{Z_a}{Z_b} = \frac{X_a}{X_b} \left (\frac{P^0_a}{P^0_b} \right) ^N </math> | ||
| Line 74: | Line 82: | ||
|align=left|= [[vapor pressure]] of pure component n | |align=left|= [[vapor pressure]] of pure component n | ||
|} | |} | ||
and <font style="vertical-align:+5%;"><math>a</math></font> and <font style="vertical-align:+15%;"><math>b</math></font> denote the more volatile and the less volatile components, respectively. | |||
==Shortcut calculations for designing | ==Shortcut calculations for designing distillation columns== | ||
There are many so-called ''shortcut'' calculation methods for designing industrial | There are many so-called ''shortcut'' calculation methods for designing industrial distillation columns. The most commonly used one is the Fenske-Underwood-Gilliland method. | ||
The Fenske equation estimates the minimum number of theoretical plates or equilibrium stages at total reflux. The [[Underwood equation]]<ref>A.J.V. Underwood (1948). ''Chemical Engineering Progress'', '''Vol. 4''':603.</ref> estimates the minimum reflux for an infinite number of theoretical equilibrium stages. The [[Gilliland method]]<ref>E.R. Gilliland (1940). ''Industrial Engineering Chemistry'', '''Vol.32''':1220.</ref> then uses Fenske's minimum plates and Underwood's minimum reflux to estimate the theoretical plates for a given distillation at a chosen reflux. | The Fenske equation estimates the minimum number of theoretical plates or equilibrium stages at total reflux. The [[Underwood equation]]<ref>A.J.V. Underwood (1948). ''Chemical Engineering Progress'', '''Vol. 4''':603.</ref> estimates the minimum reflux for an infinite number of theoretical equilibrium stages. The [[Gilliland method]]<ref>E.R. Gilliland (1940). ''Industrial Engineering Chemistry'', '''Vol.32''':1220.</ref> then uses Fenske's minimum plates and Underwood's minimum reflux to estimate the theoretical plates for a given distillation at a chosen reflux. | ||
| Line 84: | Line 93: | ||
==References== | ==References== | ||
{{reflist}} | {{reflist}}[[Category:Suggestion Bot Tag]] | ||
[[Category: | |||
Latest revision as of 06:00, 16 August 2024
The Fenske equation is used for calculating the minimum number of theoretical plates required for the separation of a binary feed stream by a distillation column that is being operated at total reflux (i.e., which means that no overhead product is being withdrawn from the column). The derivation of the Fenske equation assumes that the relative volatility is constant in the distillation column.
Theoretical plates are also often referred to as theoretical trays or equilibrium stages.
The equation was derived in 1932 by Merrell Fenske, a professor who served as the head of the chemical engineering department at the Pennsylvania State University from 1959 to 1969.[1]
When designing large-scale, continuous industrial distillation towers, it is very useful to first calculate the minimum number of theoretical plates required to obtain the desired overhead product composition.
A common version of the Fenske equation
This is one of the many different but equivalent versions of the Fenske equation:[2][3][4][5]
where:
| = minimum number of theoretical plates required at total reflux (of which the reboiler is one) | |
| = mole fraction of more volatile component in the overhead distillate | |
| = mole fraction of more volatile component in the bottoms product | |
| = average relative volatility of more volatile component to less volatile component |
For ease of expression, the more volatile and the less volatile components are commonly referred to as the light key (LK) and the heavy key (HK), respectively. Using that terminology, the above equation may be expressed as:[3]
or also:
If the relative volatility of the light key to the heavy key is constant from the column top to the column bottom, then is simply . If the relative volatility is not constant from top to bottom of the column, then the following approximation may be used:[2]
| where: | |
| = relative volatility of light key to heavy key at the top of the column | |
| = relative volatility of light key to heavy key at the bottom of the column |
The above form of the Fenske equation can be modified for use in the total reflux distillation of multi-component feeds.
Another form of the Fenske equation
Using Raoult's law and Dalton's law for a series of condensation and evaporation cycles (i.e., equilibrium stages or theoretical plates), another form of the Fenske equation is obtained for use in gas chromatography:[6]
| where: | |
| = number of equilibrium stages | |
| = mole fraction of component n in the vapor phase | |
| = mole fraction of component n in the liquid phase | |
| = vapor pressure of pure component n |
and and denote the more volatile and the less volatile components, respectively.
Shortcut calculations for designing distillation columns
There are many so-called shortcut calculation methods for designing industrial distillation columns. The most commonly used one is the Fenske-Underwood-Gilliland method.
The Fenske equation estimates the minimum number of theoretical plates or equilibrium stages at total reflux. The Underwood equation[7] estimates the minimum reflux for an infinite number of theoretical equilibrium stages. The Gilliland method[8] then uses Fenske's minimum plates and Underwood's minimum reflux to estimate the theoretical plates for a given distillation at a chosen reflux.
The estimates such as provided by the Fenske-Underwood-Gilliland shortcut calculations are most effective when used to obtain a preliminary design before following up with the use of distillation simulation software which utilize much more rigorous calculation methods.
References
- ↑ M.R. Fenske (May 24, 1932). "Fractionation of Straight-run Pennsylvania Gasoline". Industrial Engineering Chemistry 24 (5): 482-485.
- ↑ 2.0 2.1 Distillation notes (Loren Schreiber, Florida State University)
- ↑ 3.0 3.1 David S.J. Jones and Peter P. Pujado (Editors) (2006). Handbook of Petroleum Processing, First Edition. Springer. ISBN 1-4020-2819-9. Google books Search for "Fenske" to get to page 200.
- ↑ Tutorial 6: Separation Processes (J. Skilling, University of Edinburgh, Scotland)
- ↑ Maxwell, J.B. (1950). Data Book on Hydrocarbons, 1st Edition. D. Van Nostrand.
- ↑ Separation By Distillation With Quantitative Gas Chromatography (08-18-09), received as a personal communication from the Chemistry Department of the U.S. Naval Academy, which is not available online.
- ↑ A.J.V. Underwood (1948). Chemical Engineering Progress, Vol. 4:603.
- ↑ E.R. Gilliland (1940). Industrial Engineering Chemistry, Vol.32:1220.
![{\displaystyle \ N={\frac {\log \,{\bigg [}{\Big (}{\frac {X_{d}}{1-X_{d}}}{\Big )}{\Big (}{\frac {1-X_{b}}{X_{b}}}{\Big )}{\bigg ]}}{\log \,\alpha _{avg}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/06af3d7fcd0e4cac0751fb951d065cca1c4fcaf5)




![{\displaystyle \ N={\frac {\log \,{\bigg [}{\Big (}{\frac {LK_{d}}{HK_{d}}}{\Big )}{\Big (}{\frac {HK_{b}}{LK_{b}}}{\Big )}{\bigg ]}}{\log \,\alpha _{avg}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/98b652ba920414b056ca83cd3a8b1de0f0bb1c90)
![{\displaystyle \ N={\frac {\log \,{\bigg [}{\Big (}{\frac {LK_{d}}{1-LK_{d}}}{\Big )}{\Big (}{\frac {1-LK_{b}}{LK_{b}}}{\Big )}{\bigg ]}}{\log \,\alpha _{avg}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0301b1202e7c9a2987bffe2f768ddf3fcdc324c0)










