Ampicillin: Difference between revisions
imported>David E. Volk (→Brand names of ampicillin: adding ® 100 times) |
mNo edit summary |
||
| (9 intermediate revisions by 3 users not shown) | |||
| Line 20: | Line 20: | ||
'''Ampicillin''', or '''aminobenzylpenicillin''', is a broad spectrum [[antibiotic]] and a derivative of [[penicillin]]. It is used to treat infections of [[E. coli]], [[P. mirabilis]], [[enterococci]], [[Shigella]], [[S. typhosa]] and other [[Salmonella]], nonpenicillinase-producing [[N. gononhoeae]], [[H. influenzae]], [[staphylococci]], and [[streptococci]] including [[streptoc]]. It is also widely used in [[molecular biology]] labs during the over-expression of proteins in bacterial cells that are tolerant of ampicillin. This ensures that only the bacteria of interest grow in the media and produce the protein of interest. | '''Ampicillin''', or '''aminobenzylpenicillin''', is a broad spectrum [[antibiotic]] and a derivative of [[penicillin]]. It is used to treat infections of [[E. coli]], [[P. mirabilis]], [[enterococci]], [[Shigella]], [[S. typhosa]] and other [[Salmonella]], nonpenicillinase-producing [[N. gononhoeae]], [[H. influenzae]], [[staphylococci]], and [[streptococci]] including [[streptoc]]. It is also widely used in [[molecular biology]] labs during the over-expression of proteins in bacterial cells that are tolerant of ampicillin. This ensures that only the bacteria of interest grow in the media and produce the protein of interest. | ||
Ampicillin is a penicillin beta-lactam antibiotic used to eliminate susceptible, usually | Ampicillin is a penicillin beta-lactam antibiotic used to eliminate susceptible, usually Gram-positive, organisms. Like penicillin, ampicillin inhibits the last stage of bacterial cell wall synthesis by binding to specific penicillin-binding proteins in the bacterial cell wall. | ||
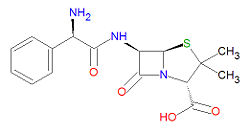
Its IUPAC chemical name is (2S,5R,6R)-6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and it has chemical formula C<sub>16</sub>H<sub>19</sub>N<sub>3</sub>O<sub>4</sub>S, giving it a molecular mass of 349.4048 g/mol. | Its IUPAC chemical name is (2S,5R,6R)-6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and it has chemical formula C<sub>16</sub>H<sub>19</sub>N<sub>3</sub>O<sub>4</sub>S, giving it a molecular mass of 349.4048 g/mol. | ||
== Synonyms of ampicillin == | == Synonyms of ampicillin == | ||
{{col-begin}} | |||
{{col-break|width=25%}} | |||
* Ampicilline | |||
* Ampicillinum | |||
* Ampicilline | |||
* Ampicillina | |||
* Ampicillin | |||
* Ampicillin Trihydrate | |||
* Ampicillin Sodium | |||
* Ampicillin Base | |||
{{col-break|width=25%}} | |||
* Ampicillin Anhydrous | |||
* Ampicillin Anhydrate | |||
* Ampicillin Acid | |||
* Ampicilina | |||
* Anhydrous Ampicillin | |||
* Bayer 5427 | |||
* D-Ampicillin | |||
* Aminobenzylpenicillin | |||
{{col-end}} | |||
== Brand names of ampicillin == | == Brand names of ampicillin == | ||
| Line 171: | Line 177: | ||
== External links == | == External links == | ||
{{CZMed}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 06:00, 10 July 2024
|
| |||||||
| ampicillin | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | beta-lactam | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Ampicillin, or aminobenzylpenicillin, is a broad spectrum antibiotic and a derivative of penicillin. It is used to treat infections of E. coli, P. mirabilis, enterococci, Shigella, S. typhosa and other Salmonella, nonpenicillinase-producing N. gononhoeae, H. influenzae, staphylococci, and streptococci including streptoc. It is also widely used in molecular biology labs during the over-expression of proteins in bacterial cells that are tolerant of ampicillin. This ensures that only the bacteria of interest grow in the media and produce the protein of interest.
Ampicillin is a penicillin beta-lactam antibiotic used to eliminate susceptible, usually Gram-positive, organisms. Like penicillin, ampicillin inhibits the last stage of bacterial cell wall synthesis by binding to specific penicillin-binding proteins in the bacterial cell wall.
Its IUPAC chemical name is (2S,5R,6R)-6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and it has chemical formula C16H19N3O4S, giving it a molecular mass of 349.4048 g/mol.
Synonyms of ampicillin
|
|
Brand names of ampicillin
|
|
|
|
External links
The most up-to-date information about Ampicillin and other drugs can be found at the following sites.
- Ampicillin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Ampicillin - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Ampicillin - Detailed information from DrugBank.