Prednisone: Difference between revisions
imported>David E. Volk (stub and structure) |
(→Indications: ridiculously long, too detailed list pared down) |
||
| (17 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Image|Prednisone structure.jpg|right|200px|Prednisone, a synthetic steroid.}} | |||
'''Prednisone''' | '''Prednisone''', also called dehydrocortisone, prednisona, or prednisonum is a synthetic anti-inflammatory [[glucocorticoid]] [[steroid]] designed as a mimic of the naturally occurring steroid [[cortisone]]. It is used to treat many inflammatory conditions. It is a prodrug that only becomes active after conversion to [[prednisolone]], a glucocorticoid agonist, in the liver. Prednisolone binds to cytoplasmic receptors to control biosynthesis of prostaglandins and leukotrienes. IUPAC name = (8S,9S,10R,13S,14S,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,12,14,15,16-octahydrocyclopenta[a]phenanthrene-3,11-dione. | ||
Prednisone was patented in 1954 and approved for medical use in the United States in 1955. It is available as a generic medication. In 2020, it was the 30th most commonly prescribed medication in the United States, with more than 19 million prescriptions.<ref>{{cite web |title=The Top 300 of 2020 |url= https://clincalc.com/DrugStats/Top300Drugs.aspx |website=ClinCalc |access-date=7 October 2022}}</ref><ref>{{cite web | title=Prednisone - Drug Usage Statistics | website=ClinCalc | url=https://clincalc.com/DrugStats/Drugs/Prednisone | access-date=7 October 2022}}</ref> | |||
== Indications == | |||
This is a prescription drug only available by the order of a licensed physician, and its use must be carefully supervised by a physician. Prednisone is use to treat a wide variety of medical issues such as allergies, carditis, systemic [[dermatomyositis]], systemic lupus erythematosus, dermatitis, Stevens-Johnson syndrome, [[psoriasis]], acute [[adrenocortical deficiency]], [[Addison's disease]], congenital [[adrenal hyperplasia]], [[hypercalcemia]] associated with neoplasms, [[Crohn's disease]], [[anemia]]s, [[erythroblastopenia]],[[thrombocytopenia]], [[bursitis]], epicondylitis, tenosynovitis, lymphocytic leukemia, [[Hodgkin's lymphoma|Hodgkin's]] or [[non-Hodgkin's lymphomas]], primary brain tumors, [[nephrotic syndrome]], [[tuberculous meningitis]], [[multiple sclerosis]], [[myasthenia gravis]], cerebral edema, [[diffuse posterior choroiditis]], [[allergic conjunctivitis]], [[Herpes zoster]] ophthalmicus, iritis, keratitis, symptomatic [[sarcoidosis]], and pulmonary tuberculosis. | |||
This is not a complete list of conditions for which prednisone is used. | |||
== Side effects== | |||
Short-term use in humans is generally safe, even at high doses; for instance, a round of five days of treatment at 50 mg a day to reduce swelling after an acute injury. However, long-term use such as that for three months at a time can produce many side effects, some serious. After long-term use, it can be dangerous to suddenly stop taking the drug, so the dose must be tapered down gradually. Life-threatening adrenal insufficiency can occur if the drug is suddenly stopped after long-term use, e.g., for four months at 25 mg a day. | |||
There is also a risk, for people with conditions such as asthma, repeatedly to need to take short courses of prednisone. The whole body's immunity is suppressed during this treatment for up to a year afterwards, so people who need more than one short course in a year may face a situation where they have less and less immunity to illnesses such as influenza. | |||
== Comparison to cortisone and prednisolone == | |||
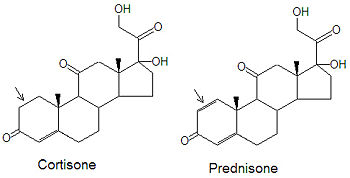
[[Image:Cortisone_Prednisone_stickfig_DEVolk.jpg|left|thumb|350px|{{#ifexist:Template:Cortisone_Prednisone_stickfig_DEVolk.jpg/credit|{{Prednisone structure.jpg/credit}}<br/>|}}Prednisone is a synthetic mimic of cortisone.]] | |||
Prednisone's structure is based on that of the naturally-occurring [[corticosteroid]] [[cortisone]]. The only difference between them is the presence of a carbon-carbon double bond in the A ring of prednisone that is not present in cortisone (note arrows in the figure). The active form of the drug, prednisolone, as the name implies ("ol"), has a hydroxyl group on ring C in place of the carbonyl group present in prednisone. | |||
== Brand names == | |||
* [[Delta-cortelan]] | |||
* [[Delta-cortisone]] | |||
* [[Delta-cortone]] | |||
* [[Delta-dome]] | |||
* [[Adasone]] | |||
* [[Ancortone]] | |||
* [[Apo-prednisone]] | |||
* [[Betapar]] | |||
* [[Bicortone]] | |||
* [[Cartancyl]] | |||
* [[Colisone]] | |||
* [[Cortan]] | |||
* [[Cortancyl]] | |||
* [[Cortidelt]] | |||
* [[Cotone]] | |||
* [[Dacorten]] | |||
* [[Dacortin]] | |||
* [[Decortancyl]] | |||
* [[Decortin]] | |||
* [[Decortisyl]] | |||
* [[Dekortin]] | |||
* [[Delcortin]] | |||
* [[Dellacort]] | |||
* [[Dellacort A]] | |||
* [[Delta Cortelan]] | |||
* [[Delta E]] | |||
* [[Deltacortene]] | |||
* [[Deltacortisone]] | |||
* [[Deltacortone]] | |||
* [[Deltasone]] | |||
* [[Deltison]] | |||
* [[Deltisona]] | |||
* [[Deltisone]] | |||
* [[Deltra]] | |||
* [[Di-Adreson]] | |||
* [[Diadreson]] | |||
* [[Econosone]] | |||
* [[Encorton]] | |||
* [[Encortone]] | |||
* [[Enkorton]] | |||
* [[Fernisone]] | |||
* [[Fiasone]] | |||
* [[Hostacortin]] | |||
* [[In-Sone]] | |||
* [[Incocortyl]] | |||
* [[Juvason]] | |||
* [[Lisacort]] | |||
* [[Me-Korti]] | |||
* [[Metacortandracin]] | |||
* [[Meticorten]] | |||
* [[Nisona]] | |||
* [[Nizon]] | |||
* [[Novoprednisone]] | |||
* [[Nurison]] | |||
* [[Orasone]] | |||
* [[Origen Prednisone]] | |||
* [[Panafcort]] | |||
* [[Panasol]] | |||
* [[Paracort]] | |||
* [[Parmenison]] | |||
* [[Pehacort]] | |||
* [[Precort]] | |||
* [[Predeltin]] | |||
* [[Prednicen-M]] | |||
* [[Prednicorm]] | |||
* [[Prednicort]] | |||
* [[Prednicot]] | |||
* [[Prednidib]] | |||
* [[Prednilonga]] | |||
* [[Prednison]] | |||
* [[Prednisone Intensol]] | |||
* [[Prednitone]] | |||
* [[Prednizon]] | |||
* [[Prednovister]] | |||
* [[Presone]] | |||
* [[Pronison]] | |||
* [[Rectodelt]] | |||
* [[Retrocortine]] | |||
* [[Servisone]] | |||
* [[Sone]] | |||
* [[Sterapred]] | |||
* [[Supercortil]] | |||
* [[Ultracorten]] | |||
* [[Ultracortene]] | |||
* [[Winpred]] | |||
* [[Wojtab]] | |||
* [[Zenadrid]] | |||
== External Links == | == External Links == | ||
{{ | {{CZMed}} | ||
==Notes==[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 22:51, 14 October 2024
Prednisone, also called dehydrocortisone, prednisona, or prednisonum is a synthetic anti-inflammatory glucocorticoid steroid designed as a mimic of the naturally occurring steroid cortisone. It is used to treat many inflammatory conditions. It is a prodrug that only becomes active after conversion to prednisolone, a glucocorticoid agonist, in the liver. Prednisolone binds to cytoplasmic receptors to control biosynthesis of prostaglandins and leukotrienes. IUPAC name = (8S,9S,10R,13S,14S,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,12,14,15,16-octahydrocyclopenta[a]phenanthrene-3,11-dione.
Prednisone was patented in 1954 and approved for medical use in the United States in 1955. It is available as a generic medication. In 2020, it was the 30th most commonly prescribed medication in the United States, with more than 19 million prescriptions.[1][2]
Indications
This is a prescription drug only available by the order of a licensed physician, and its use must be carefully supervised by a physician. Prednisone is use to treat a wide variety of medical issues such as allergies, carditis, systemic dermatomyositis, systemic lupus erythematosus, dermatitis, Stevens-Johnson syndrome, psoriasis, acute adrenocortical deficiency, Addison's disease, congenital adrenal hyperplasia, hypercalcemia associated with neoplasms, Crohn's disease, anemias, erythroblastopenia,thrombocytopenia, bursitis, epicondylitis, tenosynovitis, lymphocytic leukemia, Hodgkin's or non-Hodgkin's lymphomas, primary brain tumors, nephrotic syndrome, tuberculous meningitis, multiple sclerosis, myasthenia gravis, cerebral edema, diffuse posterior choroiditis, allergic conjunctivitis, Herpes zoster ophthalmicus, iritis, keratitis, symptomatic sarcoidosis, and pulmonary tuberculosis.
This is not a complete list of conditions for which prednisone is used.
Side effects
Short-term use in humans is generally safe, even at high doses; for instance, a round of five days of treatment at 50 mg a day to reduce swelling after an acute injury. However, long-term use such as that for three months at a time can produce many side effects, some serious. After long-term use, it can be dangerous to suddenly stop taking the drug, so the dose must be tapered down gradually. Life-threatening adrenal insufficiency can occur if the drug is suddenly stopped after long-term use, e.g., for four months at 25 mg a day.
There is also a risk, for people with conditions such as asthma, repeatedly to need to take short courses of prednisone. The whole body's immunity is suppressed during this treatment for up to a year afterwards, so people who need more than one short course in a year may face a situation where they have less and less immunity to illnesses such as influenza.
Comparison to cortisone and prednisolone
Prednisone's structure is based on that of the naturally-occurring corticosteroid cortisone. The only difference between them is the presence of a carbon-carbon double bond in the A ring of prednisone that is not present in cortisone (note arrows in the figure). The active form of the drug, prednisolone, as the name implies ("ol"), has a hydroxyl group on ring C in place of the carbonyl group present in prednisone.
Brand names
- Delta-cortelan
- Delta-cortisone
- Delta-cortone
- Delta-dome
- Adasone
- Ancortone
- Apo-prednisone
- Betapar
- Bicortone
- Cartancyl
- Colisone
- Cortan
- Cortancyl
- Cortidelt
- Cotone
- Dacorten
- Dacortin
- Decortancyl
- Decortin
- Decortisyl
- Dekortin
- Delcortin
- Dellacort
- Dellacort A
- Delta Cortelan
- Delta E
- Deltacortene
- Deltacortisone
- Deltacortone
- Deltasone
- Deltison
- Deltisona
- Deltisone
- Deltra
- Di-Adreson
- Diadreson
- Econosone
- Encorton
- Encortone
- Enkorton
- Fernisone
- Fiasone
- Hostacortin
- In-Sone
- Incocortyl
- Juvason
- Lisacort
- Me-Korti
- Metacortandracin
- Meticorten
- Nisona
- Nizon
- Novoprednisone
- Nurison
- Orasone
- Origen Prednisone
- Panafcort
- Panasol
- Paracort
- Parmenison
- Pehacort
- Precort
- Predeltin
- Prednicen-M
- Prednicorm
- Prednicort
- Prednicot
- Prednidib
- Prednilonga
- Prednison
- Prednisone Intensol
- Prednitone
- Prednizon
- Prednovister
- Presone
- Pronison
- Rectodelt
- Retrocortine
- Servisone
- Sone
- Sterapred
- Supercortil
- Ultracorten
- Ultracortene
- Winpred
- Wojtab
- Zenadrid
External Links
The most up-to-date information about Prednisone and other drugs can be found at the following sites.
- Prednisone - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Prednisone - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Prednisone - Detailed information from DrugBank.
==Notes==