Phosgene: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk |
mNo edit summary |
||
| (2 intermediate revisions by one other user not shown) | |||
| Line 6: | Line 6: | ||
Like its parent compound carbonic acid, phosgene is very reactive and decomposes in the presence of moisture to form [[carbon dioxide]] (CO2) and [[hydrochloric acid]] (HCl). The oxygen atom of water molecules act as [[nucleophile|nucleophiles]] and attack the central carbon atom. | Like its parent compound carbonic acid, phosgene is very reactive and decomposes in the presence of moisture to form [[carbon dioxide]] (CO2) and [[hydrochloric acid]] (HCl). The oxygen atom of water molecules act as [[nucleophile|nucleophiles]] and attack the central carbon atom. | ||
{{Image|Decomposition of phosgene to hydrochloric acid and carbon dioxide.jpg|left|600px|Phosgene decomposes in the presence of water to form carbon dioxide and hydrogen chloride gas.}} | |||
{{Chem infobox | {{Chem infobox | ||
|align=right | |align=right | ||
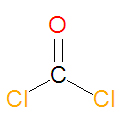
|image= | |image={{Image|Phosgene chemical structure.jpg|center|125px|Phosgene, a chemical weapon and industrial chemical}} | ||
|width=125px | |width=125px | ||
|molname=phosgene | |molname=phosgene | ||
| Line 22: | Line 22: | ||
|iupac= | |iupac= | ||
|casnumber= 75-44-5 | |casnumber= 75-44-5 | ||
}} | }}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 16:01, 3 October 2024
Phosgene, the acid chloride of carbonic acid, is an industrial chemical that was used as a chemical weapon during World War I. It is a choking gas that reacts with water to produce carbon dioxide and hydrogen chloride gas, which is corrosive. Exposure can lead to pulmonary edema and chemical pneumonitis. Phosgene has many different names, including carbon oxychloride, chloroformyl chloride, carbonyl chloride, carbonic dichloride, CG (military) and carbonyl dichloride.
Decomposition
Like its parent compound carbonic acid, phosgene is very reactive and decomposes in the presence of moisture to form carbon dioxide (CO2) and hydrochloric acid (HCl). The oxygen atom of water molecules act as nucleophiles and attack the central carbon atom.
|
| |||||||
| phosgene | |||||||
| |||||||
| Uses: | chemical weapon | ||||||
| Properties: | corrosive | ||||||
| Hazards: | corrosive, produces chlorine gas | ||||||
| |||||||