Lipostatic hypothesis: Difference between revisions
imported>Mathilda Thompson |
mNo edit summary |
||
| (18 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Image|Appetite.jpg|right|250px}} | {{Image|Appetite.jpg|right|250px}} | ||
In 1953, Kennedy <ref>Kennedy GC (1953) The role of depot fat in the hypothalamic control of food intake in the rat ''Proc R Soc Lond B Biol Sci'' 140:578-92 [http://www.jstor.org/stable/82630?seq=1]</ref> proposed what became known as ''' | In 1953, G. C. Kennedy <ref>Kennedy GC (1953) The role of depot fat in the hypothalamic control of food intake in the rat ''Proc R Soc Lond B Biol Sci'' 140:578-92 [http://www.jstor.org/stable/82630?seq=1]</ref> proposed what became known as the '''lipostatic hyothesis'''. Specifically, he postulated the existence, in the [[hypothalamus]], of a centre that was sensitive to the concentration of metabolites in the circulation, which prevented "an overall surplus of energy intake over expenditure." | ||
== Introduction == | == Introduction == | ||
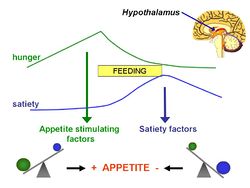
The lipostatic hypothesis describes the feedback mechanisms between adipose tissue deposition and hypothalamic signalling | The lipostatic hypothesis describes the feedback mechanisms between [[adipocyte|adipose tissue]] deposition and hypothalamic signalling that sustain 'lipostasis'. Lipostasis is achieved through the modulation and balancing of energy intake and expenditure to prevent an individual 'getting fat'. For the brain to know when there is too much adipose tissue building up, a signalling hormone is released from the adipose tissue to signal to the brain that a satiation signal must be released to stop the individual from eating. In 1994, it was discovered that this hormone released from adipose tissue, and critical in regulating the size of the body fat depot, was coded for by the ''ob'' gene and is known as [[leptin]].<ref>Zhang Y ''et al.'' (1994) Positional cloning of the mouse obese gene and its human homologue ''Nature'' 372:425-32</ref>. | ||
== What is leptin? == | == What is leptin? == | ||
Leptin is a adipocite derived hormone that circulates in the blood in proportion to whole body adipose tissue mass. In other words, except for ob/ob individuals, the more adipose tissue an individual contains, the greater the amount of leptin they have circulating in their blood. Leptin | Leptin is a adipocite derived hormone that circulates in the blood in proportion to whole body adipose tissue mass. In other words, except for ''ob-/ob-'' individuals, the more adipose tissue an individual contains, the greater the amount of leptin they have circulating in their blood. Leptin can cross the blood-brain barrier, and it acts on specific leptin receptors which are mainly located in the [[ventromedial nucleus]] (VMN) and [[arcuate nucleus]] (ARC) of the [[hypothalamus]]. The leptin signal transmits information about the size of body fat depots; in the ARC, it causes increased production of [[pro-opiomelanocortin]] (POMC), which is cleaved into functional products such as [[α-melanocyte stimulating hormoone]] (α-MSH), which then act on downstream secondary neurones leading to the release of satiation signals. | ||
Leptin was discovered as a product of the ob gene and its role in regulating food intake was identified through ob/ob | Leptin was discovered as a product of the ob gene and its role in regulating food intake was identified through ''ob/ob'' deficient mice. These mice could not produce leptin and therefore had no means of starting the feedback mechanism, as there was no signalling to the VMN and ARC of excess adipose tissue. Due to this, there is no anorexigenic response and the ob/ob mice keeps on eating due to a lack of satiation. Another mutation that further supported leptin's role in energy balance came from ''db/db'' mice. These mice have a mutation in the gene coding for leptin receptors and therefore, even though their adipose tissue produced leptin in abundance, the lack of leptin sensitive receptors also meant that there was no complete signal to the brain of excess adipose tissue. | ||
Interestingly, leptin treatment of these two different mutant mice led to drastically different results. The ob/ob mice, when treated with leptin over time, lost a vast amount of weight as leptin elicits the anorexigenic response that the mouse could not provide before. The db/db mice however had no weight reduction from the introduction of leptin, as their receptors remained insensitive to the hormone | Interestingly, leptin treatment of these two different mutant mice led to drastically different results. The ''ob/ob mice'', when treated with leptin over time, lost a vast amount of weight as leptin elicits the anorexigenic response that the mouse could not provide before. The ''db/db'' mice however had no weight reduction from the introduction of leptin, as their receptors remained insensitive to the hormone. | ||
== '''Parabolis evidence''' == | == '''Parabolis evidence''' == | ||
In the | In the 1950's it was hypothesized that there was a message from the adipose tissue to the brain, telling the brain how fat you are. Three different parabolis experiments (involving the surgical joining of two circulations of mice from a common single strain) were undertaken to test this hypothesis. The experiments involved the surgical joining of: diet-induced obese mice, and two strains of inbred obese mice: ''ob/ob'' mice and ''db/db''mice.<br /><br /> | ||
''' | ''' | ||
'''Parabolis experiments | '''Parabolis experiments'''''<br /> | ||
i)Diet induced obese mouse + normal lean mouse = weight loss in normal mouse<br /> | i)Diet-induced obese mouse + normal lean mouse = weight loss in normal mouse<br /> | ||
ii) Normal weight mouse + ob/ob obese mouse = weight loss in obese mouse<br /> | ii) Normal weight mouse + ''ob/ob'' obese mouse = weight loss in obese mouse<br /> | ||
iii)Normal weight mouse + db/db obese mouse = weight loss and death in normal mouse<br /> | iii)Normal weight mouse + ''db/db'' obese mouse = weight loss and death in normal mouse<br /> | ||
''' | '''Conclusion'''''<br /> | ||
There experiments supported the hypohesis that there is a factor in the blood which is released from adipose tissues and signals to the brain. This factor is now known to be leptin. It is the product of the ''ob'' gene and is a key regulatory metabolic hormone. | |||
''' | |||
' | |||
In experiment i) the diet-induced obese mouse has high levels of leptin in the circulation, but is leptin insensitive, therefore parabolis of the two mice means that the levels of leptin are increased in the circulation leading to weightloss in the normal mouse. In experiment ii) the ''ob/ob'' mouse has a single gene defect which means that it cannot synthesize leptin, therefore the parabolis of the mice means that the levels of leptin are increased in the circulation, this leptin can act on the hypothalamus of the ''ob/ob'' mouse leading to weight loss. In Experiment iii) the ''db/db'' mouse has mutant leptin receptors, and is obese because of its leptin insensitivity, and hence synthesizes extremely high levels of leptin. The parabolis means that the levels of leptin in the circulation are significantly increased, the leptin acts on the hypothalamus of the normal weight mouse, leading to significant weight loss and eventual death.<br /> <br /> | |||
Experiment ii) shows that a single mutation in the ob gene prevents leptin synthesis, this has also been proven to be he case in humans, and in these humans leptin therapy has been shown to significantly improve obesity disease. In contast mutations in the leptin receoptor as far less well understood and thus far | Experiment ii) shows that a single mutation in the ''ob'' gene prevents leptin synthesis, this has also been proven to be he case in humans, and in these humans leptin therapy has been shown to significantly improve obesity disease. In contast mutations in the leptin receoptor as far less well understood and thus far therapies used to treat these defects have been unsuccesful. Mutations in the leptin receptor (''db/db'') signal transduction pathway are much more common than mutations in leptin synthesis (''ob/ob'') therefore future work needs to be done to further charcterize the pathways involved in receptor signalling to ebanle the development of therapies to threat this disease. | ||
'''CONCLUSION''' | |||
It was not until the rodent parabiosis experiment in 1994, that the ob gene product leptin that is piviotal in modulation food intake and body weight was finally discovered. Leptin is released from adipose tissues into the circulation where is crosses the blood brain barrier and binds to leptin receptors in the many neurons within the hypothalamus such as the ARC, VMN and VMN. In the case of the ARC nucleus this then activates the production of the peptide mediator a-MSH which acts on further upsteam neurons which ultimately leads to the development of satiation signals. A lot of research is currently being concentrated on trying to further chacracterise these upstream secondary neurons and work out the dynamic interplay in a spatiotemporal pattern between the neurons in the hypothalamus that leads to the development of satiation signals. Although there are many other pathways involved in regulating body weight and food intake, the severe obesity resulting from the monogenic defect in the ob gene shows us what a key regulatory pathway leptin control. Obesity results in an increased risk for acquiring many further health problems such as: diabities, gall bladder disease, hypertension and breathlessness, therefore the need for a further understanding of how leptin signaling acts on the hypothalamus is imminent for the development of further therapy to treat obesity disease. | |||
==References== | ==References== | ||
{{reflist | 2}} | {{reflist | 2}}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:01, 12 September 2024
In 1953, G. C. Kennedy [1] proposed what became known as the lipostatic hyothesis. Specifically, he postulated the existence, in the hypothalamus, of a centre that was sensitive to the concentration of metabolites in the circulation, which prevented "an overall surplus of energy intake over expenditure."
Introduction
The lipostatic hypothesis describes the feedback mechanisms between adipose tissue deposition and hypothalamic signalling that sustain 'lipostasis'. Lipostasis is achieved through the modulation and balancing of energy intake and expenditure to prevent an individual 'getting fat'. For the brain to know when there is too much adipose tissue building up, a signalling hormone is released from the adipose tissue to signal to the brain that a satiation signal must be released to stop the individual from eating. In 1994, it was discovered that this hormone released from adipose tissue, and critical in regulating the size of the body fat depot, was coded for by the ob gene and is known as leptin.[2].
What is leptin?
Leptin is a adipocite derived hormone that circulates in the blood in proportion to whole body adipose tissue mass. In other words, except for ob-/ob- individuals, the more adipose tissue an individual contains, the greater the amount of leptin they have circulating in their blood. Leptin can cross the blood-brain barrier, and it acts on specific leptin receptors which are mainly located in the ventromedial nucleus (VMN) and arcuate nucleus (ARC) of the hypothalamus. The leptin signal transmits information about the size of body fat depots; in the ARC, it causes increased production of pro-opiomelanocortin (POMC), which is cleaved into functional products such as α-melanocyte stimulating hormoone (α-MSH), which then act on downstream secondary neurones leading to the release of satiation signals.
Leptin was discovered as a product of the ob gene and its role in regulating food intake was identified through ob/ob deficient mice. These mice could not produce leptin and therefore had no means of starting the feedback mechanism, as there was no signalling to the VMN and ARC of excess adipose tissue. Due to this, there is no anorexigenic response and the ob/ob mice keeps on eating due to a lack of satiation. Another mutation that further supported leptin's role in energy balance came from db/db mice. These mice have a mutation in the gene coding for leptin receptors and therefore, even though their adipose tissue produced leptin in abundance, the lack of leptin sensitive receptors also meant that there was no complete signal to the brain of excess adipose tissue.
Interestingly, leptin treatment of these two different mutant mice led to drastically different results. The ob/ob mice, when treated with leptin over time, lost a vast amount of weight as leptin elicits the anorexigenic response that the mouse could not provide before. The db/db mice however had no weight reduction from the introduction of leptin, as their receptors remained insensitive to the hormone.
Parabolis evidence
In the 1950's it was hypothesized that there was a message from the adipose tissue to the brain, telling the brain how fat you are. Three different parabolis experiments (involving the surgical joining of two circulations of mice from a common single strain) were undertaken to test this hypothesis. The experiments involved the surgical joining of: diet-induced obese mice, and two strains of inbred obese mice: ob/ob mice and db/dbmice.
Parabolis experiments
i)Diet-induced obese mouse + normal lean mouse = weight loss in normal mouse
ii) Normal weight mouse + ob/ob obese mouse = weight loss in obese mouse
iii)Normal weight mouse + db/db obese mouse = weight loss and death in normal mouse
Conclusion
There experiments supported the hypohesis that there is a factor in the blood which is released from adipose tissues and signals to the brain. This factor is now known to be leptin. It is the product of the ob gene and is a key regulatory metabolic hormone.
In experiment i) the diet-induced obese mouse has high levels of leptin in the circulation, but is leptin insensitive, therefore parabolis of the two mice means that the levels of leptin are increased in the circulation leading to weightloss in the normal mouse. In experiment ii) the ob/ob mouse has a single gene defect which means that it cannot synthesize leptin, therefore the parabolis of the mice means that the levels of leptin are increased in the circulation, this leptin can act on the hypothalamus of the ob/ob mouse leading to weight loss. In Experiment iii) the db/db mouse has mutant leptin receptors, and is obese because of its leptin insensitivity, and hence synthesizes extremely high levels of leptin. The parabolis means that the levels of leptin in the circulation are significantly increased, the leptin acts on the hypothalamus of the normal weight mouse, leading to significant weight loss and eventual death.
Experiment ii) shows that a single mutation in the ob gene prevents leptin synthesis, this has also been proven to be he case in humans, and in these humans leptin therapy has been shown to significantly improve obesity disease. In contast mutations in the leptin receoptor as far less well understood and thus far therapies used to treat these defects have been unsuccesful. Mutations in the leptin receptor (db/db) signal transduction pathway are much more common than mutations in leptin synthesis (ob/ob) therefore future work needs to be done to further charcterize the pathways involved in receptor signalling to ebanle the development of therapies to threat this disease.
CONCLUSION
It was not until the rodent parabiosis experiment in 1994, that the ob gene product leptin that is piviotal in modulation food intake and body weight was finally discovered. Leptin is released from adipose tissues into the circulation where is crosses the blood brain barrier and binds to leptin receptors in the many neurons within the hypothalamus such as the ARC, VMN and VMN. In the case of the ARC nucleus this then activates the production of the peptide mediator a-MSH which acts on further upsteam neurons which ultimately leads to the development of satiation signals. A lot of research is currently being concentrated on trying to further chacracterise these upstream secondary neurons and work out the dynamic interplay in a spatiotemporal pattern between the neurons in the hypothalamus that leads to the development of satiation signals. Although there are many other pathways involved in regulating body weight and food intake, the severe obesity resulting from the monogenic defect in the ob gene shows us what a key regulatory pathway leptin control. Obesity results in an increased risk for acquiring many further health problems such as: diabities, gall bladder disease, hypertension and breathlessness, therefore the need for a further understanding of how leptin signaling acts on the hypothalamus is imminent for the development of further therapy to treat obesity disease.