Count Rumford: Difference between revisions
imported>Paul Wormer No edit summary |
mNo edit summary |
||
| (44 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | |||
'''Count Rumford''' | {{Image|Sir Benjamin Thompson, Count Rumford.jpg|right|266px|Colonel Benjamin Thompson, FRS, in British army uniform. Painting by Thomas Gainsborough 1783}} | ||
'''Count Rumford''' (born '''Benjamin Thompson''', 1753–1814) was an American born soldier, statesman, scientist, inventor and social reformer. He is most famous for his scientific work, publishing over seventy papers mostly related to [[light]], [[heat]], food, and [[cooking]]. During his time in the military, his work with cannons led him to discover that [[friction]] can generate an inexhaustible amount of heat, which challenged the [[caloric theory]] that regarded heat as a substance. | |||
Thompson's other career achievements include attaining the rank of colonel in the British army, being elected a Fellow of the [[Royal Society]] in England in 1779, being knighted by [[King George III]] in 1781, and being ennobled to Count of the [[Holy Roman Empire]] by the Duke of Bavaria in 1792. | |||
knighted by King George III in 1781 | |||
Roman Empire by the Duke of Bavaria in 1792. | |||
==Biography== | ==Biography== | ||
===Early life=== | |||
Benjamin Thompson was born on March 26, 1753 in Woburn, Massachusetts. His father was a farmer who died when | |||
Benjamin was twenty months old. He attended grade school in Woburn, where he demonstrated an aptitude for [[mathematics]]. At age thirteen, he left school and was apprenticed as a shop assistant. Thompson recognized early on that this was not a career he wished to pursue and sought to further his education on this own. In his spare time he studied [[chemistry]], [[physics]], and [[anatomy]] under the tutelage of community elders. He demonstrated a particular interest in science and technology, attempting to replicate [[Benjamin Franklin]]'s kite experiment, make fireworks, and build a perpetual motion machine. When he turned eighteen he decided to try his hand at teaching and was invited to run a school in Concord. It was there that he met Sarah Walker, a wealthy window who was eleven years his senior, and married her in November 1772. His wife introduced him to the ruling circles of New England where he made such an impression that at the age of twenty he received a major's commission in the second New Hampshire Regiment, even though he had no military experience whatsoever. | |||
===American Revolutionary War=== | |||
During the [[American Revolution|American war of Independence]] (1775-1783) Thompson was a [[Loyalists|loyalist]], choosing to remain loyal to the British. This made him unpopular in New Hampshire and he fled to Boston, where he offered his services to General [[Thomas Gage]] and served as a spy on the [[Continental Army]]. Thompson proved to be skilled at his craft, transmitting letters using invisible ink. When Boston was taken by the Americans on March 17, 1776 Thompson fled to England leaving his wife and daughter behind. He never saw his wife again, but his daughter Sarah joined him in Europe twenty years later. | |||
Upon his arrival in England, he presented himself to Lord [[George Germain]], the Secretary of State for the Colonies in the cabinet of [[Frederic North|Lord North]], bearing letters of recommendation from New Hampshire Governor John Wentworth and General [[William Howe]]. The meeting resulted in a lifelong friendship. Thompson provided Germain with accurate information about the war and in return Germain gave Thompson an entry into English society. Germain's influence with King [[George III]] procured Thompson an appointment as Secretary of the Province of Georgia, which although a meaningless sinecure, brought him £100 per annum. This position left him enough time to perform an important series of scientific experiments on the properties of [[gunpowder]]. He investigated whether humid—water containing—gunpowder was more forceful than dry powder, as was often thought. He adapted a very clever experimental setup, based on ideas first put forth by Benjamin Robins in ''New Principles of Gunnery'' in 1742, and improved upon by mathematician Charles Hutton in 1778, with both the gun and its heavy wooden target as free-swinging pendulums, so that he could measure accurately the forces of recoil and impact. He found that the vaporization of water did not improve the quality of gunpowder, or in other words, dry gunpowder works better. The scientific worth of this research was recognized and Thompson was made a Fellow of the Royal Society at the age of twenty-seven. A year later he published his findings in a 99-page paper entitled ''New Experiments upon Gunpowder'', in the 1781 volume of the ''Philosophical Transactions of the Royal Society''. | |||
Thompson climbed rapidly in English society and in 1779 he was appointed deputy to the Inspector General of Provincial Forces. Thompson's sole responsibility was to provide clothing and other stores for the British colonial armed forces. The position turned out to be very lucrative, enabling him to take advantage of the fact that silk, widely used in uniforms, is hygroscopic; dry silk bought in London would absorb 10 percent of its weight in the journey across the Atlantic, which allowed him to make a considerable profit. It was reported that Thompson made about £7000 per year, which led to accusations of corruption. However, these charges appear to have been ignored and in 1780 he was promoted to Under Secretary of State for the Colonies. | |||
With the war in America going poorly, as well as his friendship with the unpopular Lord Germain, made Thompson's political career uncertain. Using the wealth he had recently accumulated, he bought himself an appointment as lieutenant-colonel of the King's American dragoons. Abandoning his post as Under Secretary, he sailed for America and arrived more than two months later in Charleston, on December 29, 1781. From there, he traveled to New York where he started to enlist men for his regiment and by August 1782 the King's American Dragoons were ready for service. The Dragoons were involved in several skirmishes. However, soon after (on November 30, 1782) the hostilities ceased and the [[Treaty of Paris (1783)|Treaty of Paris]] was signed on September 3, 1783. | |||
The end of the war left Thompson with an uncertain future. Although Britain was at peace, he decided his interests lied in continuing his military career and central Europe seemed to offer him the best chances. In an attempt to enhance his image he used his contacts in London to receive a promotion to full colonel<ref>Thompson retired at the rank of full colonel, which awarded him half-pay for the rest of his life after only serving in the British army for sixteen months,</ref> and obtained a letter of recommendation from Lord North. | |||
===Bavarian years=== | |||
On his way to Vienna to fight the [[Ottomans]], Thompson stopped in Strasbourg where Prince Maximilian von Zweibrücken of [[Bavaria]] was reviewing his troops. The two men became friends, which was an amazing feat given the class consciousness and the importance attached to noble birth in eighteenth century Europe. Through Maximilian's influence Thompson became colonel in the Bavarian army and aide-de-camp to the [[Prince-Elector]] (German: Kurfürst) of Bavaria, Karl II (Karl Philipp Theodor, 1724 – 1799), as well as tutor to his illegitimate son. | |||
to | |||
Before Thompson could accept this position, he had to return to England to ask for permission as he was still a servant of King George III. The English government saw his appointment as an excellent chance to gain influence in Bavaria (an independent state in the [[Holy Roman Empire]]), and not only granted him permission but also knighted him. Thompson would stay in Bavaria for twelve years climbing through the ranks. Thompson's first four years were spent absorbing the culture of Bavaria and learning to speak German and French. He observed a country that was disorganized, full of corruption, and ripe for reform. On February 7, 1788 he submitted a plan to the Elector outlining his plans for reorganizing the military. The plan was well received and Thompson was promoted to rank of major-general and given the the positions of Minister of War, Minister of Police, and Chamberlain to the Court. | |||
in | |||
Prior to Thompson's reforms, the Bavarian army was in disarray, with about a quarter of the men being officers. Although Bavaria is completely landlocked and had no navy, it did have a great admiral. The common soldiers were mainly conscripted, unwilling, underpaid, and untrained peasants. Full of energy Thompson tackled these problems. Some of his sweeping reforms included increasing the pay of the soldiers and setting up schools to educate them, establishing a military academy for the training of officers, softening disciplinary procedures, eliminating army service as a criminal punishment, and introducing scientific principles in nutrition, so that the men were fed well (and at less cost). He also reorganized the manufacturing of cannon. | |||
Thompson's next round of reforms were targeted at the impoverished population. It was estimated that about five percent of Bavarians lived on begging. Gangs of beggars were highly territorial and operated in a [[Mafia]]-like manner. Thompson's plan was to round them all up and employ them in workhouses making military uniforms. On New Year's Day 1790, the military arrested all the beggars they could find in Munich. This plan worked to a large extent since Thompson took care that the regime in the workhouses was benign and inmates were trained in a wide variety of skills and paid on a piecework basis. The social experiment was a success and many beggars were reabsorbed into society. | |||
in | |||
Another great success of Thompson's, inspired by the [[Kew Gardens]] in London, was the construction of the [[English Garden]] on a patch of marshy ground on the banks of the Isar River. The Garden, which was the finest park in Europe when it opened to the public in 1791, still exists in the city of Munich today. | |||
of | |||
the | |||
Although Thompson did not have the best relationship with the people of Bavaria, his successful reform programs solidified his popularity with the Elector. On May 9,1792 Karl II promoted him to a the rank and dignity of the Imperial Counts of the Holy Roman Empire. Sir Benjamin Thompson, Fellow of the Royal Society, chose the title Count Rumford, after the New Hampshire town,<ref>In 1765 renamed to Concord. The city became state capital in 1808.</ref> which he had left behind sixteen years ago. | |||
Politically, the situation was shifting in the wrong direction for Count Rumford at the end of 1792. During his energetic enterprises he had made many enemies in Munich and, moreover, his protector, Karl Theodor (Karl II), was aging and losing power. In March 1793, the Count, with his health declining, found it opportune to be out of sight for a while and was granted permission to travel to Italy where he spent the next sixteen months. When Rumford returned to Bavaria in the summer of 1794, the political situation was worsening. While in Italy, Rumford had met [[Alessandro Volta]] and Charles Bladgen,the secretary of the Royal Society, who inspired him to spend more time on science. The Elector granted the Count six months leave of absence to pursue his scientific endeavors, which Rumford decided to spend in London. | |||
===Scientific focus=== | |||
Rumford | Back in England, Count Rumford began by writing several essays. Some were chronicles of his past achievements with combating poverty in Bavaria, but he did cover new ground with his essay on improving chimney design. Rumford's design was based on his understanding of convection and the result was a fireplace that prevented clouds of smoke from entering the room. In March 1796, Rumford endowed two prize medals, one to the Royal Society in London(the [[Rumford Medal]]) and the other to the [[American Academy of Arts and Sciences]] in Boston (the [[Rumford Prize]]). The medals reward inventors for discoveries in heating and lighting, and are still awarded today. His time in London also afforded him a reunion with his daughter, Sally (Sarah). | ||
and | |||
In August 1796, Count Rumford was summoned to return to Munich by Karl II due to the threats posed by the war between France and Austria. Rumford was quickly given control of the army, as the powers ruling Munich thought that a foreigner would be a convenient scapegoat if the town were invaded. The Austrians set up camp on the north side of town and the French to the west. Each army was determined to occupy Munich, but Rumford, shuttling between the camps and playing for time, managed to avoid triggering any conflict until the French were pulled out following the defeat of another French army. Rumford's non-violent defense of Munich made him a national hero. A monument to his success was erected in the English Garden, a street was named after him, and his daughter was made a Countess. When the danger was over, Rumford found time to do his famous cannon boring experiments that established the [[thermodynamics|thermodynamic]] connection between heat and work. However, Rumford had made a proposal to increase the size of Bavaria's army in order to decrease their dependence on Austria, which angered the Austrian Emperor. In order to maintain the peace, the Elector, appointed Count Rumford to Minister Plenipotentiary to the Court of St James (Bavarian Ambassador in London), to get Rumford out of the way. | |||
Rumford arrived back in London on September 19, 1798 to find that George III refused to accept him as ambassador of a foreign country. Rumford's days as a politician were finished. He expressed a desire to return to the United States in 1799 and was eagerly sought by the Americans (who needed help in fighting the [[Quasi-War]] with France).<ref>James E. Bradley, "The Reprieve of a Loyalist: Count Rumford's Invitation Home." ''New England Quarterly'' 1974 47(3): 368-385. ISSN 0028-4866 [http://links.jstor.org/sici?sici=0028-4866(197409)47%3A3%3C368%3ATROALC%3E2.0.CO%3B2-K in Jstor]</ref> Rumford eventually decided to stay in London because he was involved in establishing a combined museum, research and | |||
educational institute. In January 1800, the institute was granted the Royal seal, becoming the [[Royal Institution of Great Britain]]. Under the direction of [[Humphry Davy]], who soon replaced the first director [[Thomas Garnett]], the institution was a huge success in promoting the public understanding of the natural sciences. | |||
===Later years=== | |||
Soon after appointing Davy, Rumford visited Munich to pay his respects to new Elector, his old friend Maximilian von Zweibrücken, who took the name Maximilian IV (Shortly before the end of the Holy Roman Empire in 1806 he became King Maximilian I of Bavaria). On his way back to England, Rumford stopped in Paris where he renewed his acquaintance with the widow, [[Marie-Anne Paulze]], of the great chemist [[Lavoisier|Antoine Lavoisier]] who was decapitated during the [[Reign of Terror]]. They started an affair and in 1804 the couple settled in a house in Paris. In October 1805, after Rumford received proof that his American wife was deceased, the couple married. Although they had had an affair for almost five years, they found that they were incompatible and separated after a couple of years. Rumford found a house in Auteuil, four miles from the center of Paris, where he lived from 1808 until his death on August 21, 1814 at the age of sixty-one. | |||
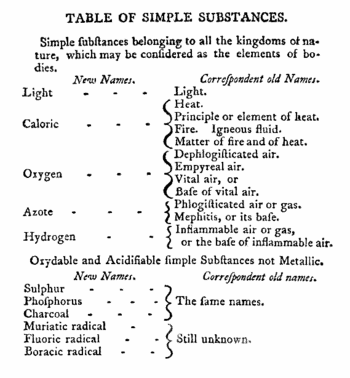
[[Image:Lavoisiers elements.gif|left|thumb|350px|{{#ifexist:Template:Lavoisiers elements.gif/credit|{{Lavoisiers elements.gif/credit}}<br/>|}}Part of table from English translation of Ref.<ref> | |||
[[Antoine Lavoisier]] ''Traité élémentaire de chimie'', 2 vols. Chez Cuchet, Paris (1789). Translated from the | |||
French by Robert Kerr, ''Elements of Chemistry'', 4th edition. William Creech, Edinburgh: (1790) p. 175</ref>]] | |||
==Rumford's science== | ==Rumford's science== | ||
As has been mentioned above, Thompson's research on the | |||
explosive power of gunpowder obtained him a membership of the Royal | |||
Society. In the second half of the 1780s, while he was restructuring | |||
the Bavarian army, Colonel Thompson's interest in army uniforms led him | |||
to the study of the conduction of heat. He discovered that convection | |||
of air is an important carrier of heat, but that still air is a good | |||
insulator. The Royal Society awarded him the [[Copley medal]] for this | |||
work. Thompson was the kind of scientist who loved to apply his | |||
findings to practical applications. In this case it lead him to the | |||
design of fluffy woollen army uniforms for the winter and cotton | |||
uniforms for the summer. | |||
At the end of the eighteenth century there were two competing theories of [[Energy#Heat|heat]] (flow of energy from hot to cold). [[Antoine Lavoisier]] held that heat is a fluid and is better called caloric, just | |||
as [[Oxygen#History|dephlogisticated air]] is better called oxygen; see | |||
the reproduction of part of Lavoisier's book on the left. Another | |||
theory—the now generally accepted kinetic theory—held that heat is a form of motion of the particles constituting matter. The particles of a hot body move more vehemently than the ones of a cold body. Count | |||
Rumford adhered to the latter theory. If heat/caloric were a fluid of | |||
which a finite amount would be contained in matter, it would be possible | |||
to exhaust it, in the same way as burning a log of wood gives off a | |||
finite amount of heat—ashes are deplete of caloric. | |||
Rumford demonstrated in Munich around 1797, shortly after he saved the city from | |||
plunder by either the French or the Austrian army, that the heat | |||
generated by friction is inexhaustible. To show this, he bored a brass | |||
cylinder, waste product of the Munich cannon foundry, with a blunt drill | |||
bit. The setup was such that the cylinder was rotated by two horses who | |||
did their work via a system of gears, while the drill bit was stationairy. | |||
The friction generated an enormous amount of heat which Rumford was able | |||
to measure quantitatively by heating water. The conclusion was that | |||
heat was inexhaustible: as long as the horses kept working and the | |||
drill bit was boring the cylinder, heat continued being generated. | |||
Rumford could quickly bring large quantities of water to boil without | |||
any fire, to the great astonishment of visitors whom he showed the | |||
experiment. But Rumford liked to point out that this is not the most | |||
efficient way to heat water, more efficient would be, he said, the | |||
burning of the hay that fed the horses. With this remark he was on the | |||
edge of understanding that energy is conserved (first law of | |||
[[thermodynamics]]) and that energy can be converted from one form to | |||
another. It took about fifty more years before this principle was | |||
understood by men such as [[Julius Mayer]] and [[James Joule]]. | |||
Work is a form of [[energy]]. For instance, the work necessary to lift | |||
a kilogram from the surface of the Earth to a height of one meter is | |||
9.81 [[joule]] (see [[Acceleration due to gravity]]). Energy used to | |||
have also a definition in terms of heat: one kilocalorie (kcal) of | |||
energy can heat a liter water from 14.5 to 15.5 <sup>0</sup>C. Rumford | |||
was the first to see the equivalence of work and heat and was able to express | |||
an amount of heat in a corresponding amount of work. From his cannon-boring | |||
experiments he determined that 1 cal = 5.60 joule<ref>Later measurements | |||
refined this number to 4.184, and we are now so certain of the | |||
equivalence of work and heat that there is only one [[SI]] unit of | |||
energy, the joule. The calorie is ''defined'' as 4.184 joule.</ref> | |||
A further blow to the caloric theory was when Rumford determined that | |||
heat is weightless (the physicist says massless). This can be | |||
expected if heat is a motion of constituting particles; if it is a fluid | |||
it can be expected to have some weight. Rumford filled three identical | |||
bottles with equal weights of water, mercury and alcohol, all of | |||
temperature 16 <sup>0</sup>C. Since on cooling down the three | |||
substances give off widely varying amounts of heat (the compounds have | |||
very different [[heat capacity]]), it could be expected that after | |||
cooling down to −1 <sup>0</sup>C, the bottles have different | |||
weight. Such a "null experiment" can be executed with great accuracy, | |||
and this is what Rumford did, finding no weight difference whatsoever. | |||
Rumford presented his findings in a communication entitled ''Enquiry Concerning the Source of Heat Which Is Excited by Friction'', to the Royal Society in 1798. Since then the idea that heat is a form of motion is generally accepted, although some of Rumford's contemporaries such as [[Laplace]] and [[John Dalton]] died unconvinced of the kinetic theory. | |||
Rumford was also an inventor in the field of lighting, heating and cooking. | |||
He developed (around 1806) an oil lamp that gave six times more light than the existing [[Argand lamp]], although the latter lamp, invented in 1780, was already a great improvement on the candle light in use before that. In order to make sure that developments on his lamps were really improvements, Rumford invented a photometer by which the yields of lamps could be assessed objectively. | |||
{{Image|Rumford fireplace.jpg|right|350px|The Rumford fireplace, which is still used today.}} | |||
The Rumford fireplace was briefly mentioned above. Further improvements were in the shape of the chimney and the fireplace itself. The Rumford fireplace radiated more heat for less fuel than the old fireplaces. The Rumford fireplace was a great success and is still on the market—mainly for the purpose of restoring old houses into their original state. | |||
Another great success was his invention of an embryonic kitchen range, not unlike those used today, together with what came to be called a Rumford roaster. In the kitchen range up to twelve separate fireplaces could be built in and they were designed to heat special pots, pans, kettles, etc. all invented by Rumford. The Rumford roaster was designed for cooking meat that hitherto had been done on a spit over an open fire. Rumford also revolutionized cooking for armies in the field. | |||
==References and notes == | |||
<references />[[Category:Suggestion Bot Tag]] | |||
[[Category: | |||
Latest revision as of 11:01, 2 August 2024
Count Rumford (born Benjamin Thompson, 1753–1814) was an American born soldier, statesman, scientist, inventor and social reformer. He is most famous for his scientific work, publishing over seventy papers mostly related to light, heat, food, and cooking. During his time in the military, his work with cannons led him to discover that friction can generate an inexhaustible amount of heat, which challenged the caloric theory that regarded heat as a substance.
Thompson's other career achievements include attaining the rank of colonel in the British army, being elected a Fellow of the Royal Society in England in 1779, being knighted by King George III in 1781, and being ennobled to Count of the Holy Roman Empire by the Duke of Bavaria in 1792.
Biography
Early life
Benjamin Thompson was born on March 26, 1753 in Woburn, Massachusetts. His father was a farmer who died when Benjamin was twenty months old. He attended grade school in Woburn, where he demonstrated an aptitude for mathematics. At age thirteen, he left school and was apprenticed as a shop assistant. Thompson recognized early on that this was not a career he wished to pursue and sought to further his education on this own. In his spare time he studied chemistry, physics, and anatomy under the tutelage of community elders. He demonstrated a particular interest in science and technology, attempting to replicate Benjamin Franklin's kite experiment, make fireworks, and build a perpetual motion machine. When he turned eighteen he decided to try his hand at teaching and was invited to run a school in Concord. It was there that he met Sarah Walker, a wealthy window who was eleven years his senior, and married her in November 1772. His wife introduced him to the ruling circles of New England where he made such an impression that at the age of twenty he received a major's commission in the second New Hampshire Regiment, even though he had no military experience whatsoever.
American Revolutionary War
During the American war of Independence (1775-1783) Thompson was a loyalist, choosing to remain loyal to the British. This made him unpopular in New Hampshire and he fled to Boston, where he offered his services to General Thomas Gage and served as a spy on the Continental Army. Thompson proved to be skilled at his craft, transmitting letters using invisible ink. When Boston was taken by the Americans on March 17, 1776 Thompson fled to England leaving his wife and daughter behind. He never saw his wife again, but his daughter Sarah joined him in Europe twenty years later.
Upon his arrival in England, he presented himself to Lord George Germain, the Secretary of State for the Colonies in the cabinet of Lord North, bearing letters of recommendation from New Hampshire Governor John Wentworth and General William Howe. The meeting resulted in a lifelong friendship. Thompson provided Germain with accurate information about the war and in return Germain gave Thompson an entry into English society. Germain's influence with King George III procured Thompson an appointment as Secretary of the Province of Georgia, which although a meaningless sinecure, brought him £100 per annum. This position left him enough time to perform an important series of scientific experiments on the properties of gunpowder. He investigated whether humid—water containing—gunpowder was more forceful than dry powder, as was often thought. He adapted a very clever experimental setup, based on ideas first put forth by Benjamin Robins in New Principles of Gunnery in 1742, and improved upon by mathematician Charles Hutton in 1778, with both the gun and its heavy wooden target as free-swinging pendulums, so that he could measure accurately the forces of recoil and impact. He found that the vaporization of water did not improve the quality of gunpowder, or in other words, dry gunpowder works better. The scientific worth of this research was recognized and Thompson was made a Fellow of the Royal Society at the age of twenty-seven. A year later he published his findings in a 99-page paper entitled New Experiments upon Gunpowder, in the 1781 volume of the Philosophical Transactions of the Royal Society.
Thompson climbed rapidly in English society and in 1779 he was appointed deputy to the Inspector General of Provincial Forces. Thompson's sole responsibility was to provide clothing and other stores for the British colonial armed forces. The position turned out to be very lucrative, enabling him to take advantage of the fact that silk, widely used in uniforms, is hygroscopic; dry silk bought in London would absorb 10 percent of its weight in the journey across the Atlantic, which allowed him to make a considerable profit. It was reported that Thompson made about £7000 per year, which led to accusations of corruption. However, these charges appear to have been ignored and in 1780 he was promoted to Under Secretary of State for the Colonies.
With the war in America going poorly, as well as his friendship with the unpopular Lord Germain, made Thompson's political career uncertain. Using the wealth he had recently accumulated, he bought himself an appointment as lieutenant-colonel of the King's American dragoons. Abandoning his post as Under Secretary, he sailed for America and arrived more than two months later in Charleston, on December 29, 1781. From there, he traveled to New York where he started to enlist men for his regiment and by August 1782 the King's American Dragoons were ready for service. The Dragoons were involved in several skirmishes. However, soon after (on November 30, 1782) the hostilities ceased and the Treaty of Paris was signed on September 3, 1783.
The end of the war left Thompson with an uncertain future. Although Britain was at peace, he decided his interests lied in continuing his military career and central Europe seemed to offer him the best chances. In an attempt to enhance his image he used his contacts in London to receive a promotion to full colonel[1] and obtained a letter of recommendation from Lord North.
Bavarian years
On his way to Vienna to fight the Ottomans, Thompson stopped in Strasbourg where Prince Maximilian von Zweibrücken of Bavaria was reviewing his troops. The two men became friends, which was an amazing feat given the class consciousness and the importance attached to noble birth in eighteenth century Europe. Through Maximilian's influence Thompson became colonel in the Bavarian army and aide-de-camp to the Prince-Elector (German: Kurfürst) of Bavaria, Karl II (Karl Philipp Theodor, 1724 – 1799), as well as tutor to his illegitimate son.
Before Thompson could accept this position, he had to return to England to ask for permission as he was still a servant of King George III. The English government saw his appointment as an excellent chance to gain influence in Bavaria (an independent state in the Holy Roman Empire), and not only granted him permission but also knighted him. Thompson would stay in Bavaria for twelve years climbing through the ranks. Thompson's first four years were spent absorbing the culture of Bavaria and learning to speak German and French. He observed a country that was disorganized, full of corruption, and ripe for reform. On February 7, 1788 he submitted a plan to the Elector outlining his plans for reorganizing the military. The plan was well received and Thompson was promoted to rank of major-general and given the the positions of Minister of War, Minister of Police, and Chamberlain to the Court.
Prior to Thompson's reforms, the Bavarian army was in disarray, with about a quarter of the men being officers. Although Bavaria is completely landlocked and had no navy, it did have a great admiral. The common soldiers were mainly conscripted, unwilling, underpaid, and untrained peasants. Full of energy Thompson tackled these problems. Some of his sweeping reforms included increasing the pay of the soldiers and setting up schools to educate them, establishing a military academy for the training of officers, softening disciplinary procedures, eliminating army service as a criminal punishment, and introducing scientific principles in nutrition, so that the men were fed well (and at less cost). He also reorganized the manufacturing of cannon.
Thompson's next round of reforms were targeted at the impoverished population. It was estimated that about five percent of Bavarians lived on begging. Gangs of beggars were highly territorial and operated in a Mafia-like manner. Thompson's plan was to round them all up and employ them in workhouses making military uniforms. On New Year's Day 1790, the military arrested all the beggars they could find in Munich. This plan worked to a large extent since Thompson took care that the regime in the workhouses was benign and inmates were trained in a wide variety of skills and paid on a piecework basis. The social experiment was a success and many beggars were reabsorbed into society.
Another great success of Thompson's, inspired by the Kew Gardens in London, was the construction of the English Garden on a patch of marshy ground on the banks of the Isar River. The Garden, which was the finest park in Europe when it opened to the public in 1791, still exists in the city of Munich today.
Although Thompson did not have the best relationship with the people of Bavaria, his successful reform programs solidified his popularity with the Elector. On May 9,1792 Karl II promoted him to a the rank and dignity of the Imperial Counts of the Holy Roman Empire. Sir Benjamin Thompson, Fellow of the Royal Society, chose the title Count Rumford, after the New Hampshire town,[2] which he had left behind sixteen years ago.
Politically, the situation was shifting in the wrong direction for Count Rumford at the end of 1792. During his energetic enterprises he had made many enemies in Munich and, moreover, his protector, Karl Theodor (Karl II), was aging and losing power. In March 1793, the Count, with his health declining, found it opportune to be out of sight for a while and was granted permission to travel to Italy where he spent the next sixteen months. When Rumford returned to Bavaria in the summer of 1794, the political situation was worsening. While in Italy, Rumford had met Alessandro Volta and Charles Bladgen,the secretary of the Royal Society, who inspired him to spend more time on science. The Elector granted the Count six months leave of absence to pursue his scientific endeavors, which Rumford decided to spend in London.
Scientific focus
Back in England, Count Rumford began by writing several essays. Some were chronicles of his past achievements with combating poverty in Bavaria, but he did cover new ground with his essay on improving chimney design. Rumford's design was based on his understanding of convection and the result was a fireplace that prevented clouds of smoke from entering the room. In March 1796, Rumford endowed two prize medals, one to the Royal Society in London(the Rumford Medal) and the other to the American Academy of Arts and Sciences in Boston (the Rumford Prize). The medals reward inventors for discoveries in heating and lighting, and are still awarded today. His time in London also afforded him a reunion with his daughter, Sally (Sarah).
In August 1796, Count Rumford was summoned to return to Munich by Karl II due to the threats posed by the war between France and Austria. Rumford was quickly given control of the army, as the powers ruling Munich thought that a foreigner would be a convenient scapegoat if the town were invaded. The Austrians set up camp on the north side of town and the French to the west. Each army was determined to occupy Munich, but Rumford, shuttling between the camps and playing for time, managed to avoid triggering any conflict until the French were pulled out following the defeat of another French army. Rumford's non-violent defense of Munich made him a national hero. A monument to his success was erected in the English Garden, a street was named after him, and his daughter was made a Countess. When the danger was over, Rumford found time to do his famous cannon boring experiments that established the thermodynamic connection between heat and work. However, Rumford had made a proposal to increase the size of Bavaria's army in order to decrease their dependence on Austria, which angered the Austrian Emperor. In order to maintain the peace, the Elector, appointed Count Rumford to Minister Plenipotentiary to the Court of St James (Bavarian Ambassador in London), to get Rumford out of the way.
Rumford arrived back in London on September 19, 1798 to find that George III refused to accept him as ambassador of a foreign country. Rumford's days as a politician were finished. He expressed a desire to return to the United States in 1799 and was eagerly sought by the Americans (who needed help in fighting the Quasi-War with France).[3] Rumford eventually decided to stay in London because he was involved in establishing a combined museum, research and educational institute. In January 1800, the institute was granted the Royal seal, becoming the Royal Institution of Great Britain. Under the direction of Humphry Davy, who soon replaced the first director Thomas Garnett, the institution was a huge success in promoting the public understanding of the natural sciences.
Later years
Soon after appointing Davy, Rumford visited Munich to pay his respects to new Elector, his old friend Maximilian von Zweibrücken, who took the name Maximilian IV (Shortly before the end of the Holy Roman Empire in 1806 he became King Maximilian I of Bavaria). On his way back to England, Rumford stopped in Paris where he renewed his acquaintance with the widow, Marie-Anne Paulze, of the great chemist Antoine Lavoisier who was decapitated during the Reign of Terror. They started an affair and in 1804 the couple settled in a house in Paris. In October 1805, after Rumford received proof that his American wife was deceased, the couple married. Although they had had an affair for almost five years, they found that they were incompatible and separated after a couple of years. Rumford found a house in Auteuil, four miles from the center of Paris, where he lived from 1808 until his death on August 21, 1814 at the age of sixty-one.
Rumford's science
As has been mentioned above, Thompson's research on the explosive power of gunpowder obtained him a membership of the Royal Society. In the second half of the 1780s, while he was restructuring the Bavarian army, Colonel Thompson's interest in army uniforms led him to the study of the conduction of heat. He discovered that convection of air is an important carrier of heat, but that still air is a good insulator. The Royal Society awarded him the Copley medal for this work. Thompson was the kind of scientist who loved to apply his findings to practical applications. In this case it lead him to the design of fluffy woollen army uniforms for the winter and cotton uniforms for the summer.
At the end of the eighteenth century there were two competing theories of heat (flow of energy from hot to cold). Antoine Lavoisier held that heat is a fluid and is better called caloric, just as dephlogisticated air is better called oxygen; see the reproduction of part of Lavoisier's book on the left. Another theory—the now generally accepted kinetic theory—held that heat is a form of motion of the particles constituting matter. The particles of a hot body move more vehemently than the ones of a cold body. Count Rumford adhered to the latter theory. If heat/caloric were a fluid of which a finite amount would be contained in matter, it would be possible to exhaust it, in the same way as burning a log of wood gives off a finite amount of heat—ashes are deplete of caloric.
Rumford demonstrated in Munich around 1797, shortly after he saved the city from plunder by either the French or the Austrian army, that the heat generated by friction is inexhaustible. To show this, he bored a brass cylinder, waste product of the Munich cannon foundry, with a blunt drill bit. The setup was such that the cylinder was rotated by two horses who did their work via a system of gears, while the drill bit was stationairy. The friction generated an enormous amount of heat which Rumford was able to measure quantitatively by heating water. The conclusion was that heat was inexhaustible: as long as the horses kept working and the drill bit was boring the cylinder, heat continued being generated. Rumford could quickly bring large quantities of water to boil without any fire, to the great astonishment of visitors whom he showed the experiment. But Rumford liked to point out that this is not the most efficient way to heat water, more efficient would be, he said, the burning of the hay that fed the horses. With this remark he was on the edge of understanding that energy is conserved (first law of thermodynamics) and that energy can be converted from one form to another. It took about fifty more years before this principle was understood by men such as Julius Mayer and James Joule.
Work is a form of energy. For instance, the work necessary to lift a kilogram from the surface of the Earth to a height of one meter is 9.81 joule (see Acceleration due to gravity). Energy used to have also a definition in terms of heat: one kilocalorie (kcal) of energy can heat a liter water from 14.5 to 15.5 0C. Rumford was the first to see the equivalence of work and heat and was able to express an amount of heat in a corresponding amount of work. From his cannon-boring experiments he determined that 1 cal = 5.60 joule[5]
A further blow to the caloric theory was when Rumford determined that heat is weightless (the physicist says massless). This can be expected if heat is a motion of constituting particles; if it is a fluid it can be expected to have some weight. Rumford filled three identical bottles with equal weights of water, mercury and alcohol, all of temperature 16 0C. Since on cooling down the three substances give off widely varying amounts of heat (the compounds have very different heat capacity), it could be expected that after cooling down to −1 0C, the bottles have different weight. Such a "null experiment" can be executed with great accuracy, and this is what Rumford did, finding no weight difference whatsoever.
Rumford presented his findings in a communication entitled Enquiry Concerning the Source of Heat Which Is Excited by Friction, to the Royal Society in 1798. Since then the idea that heat is a form of motion is generally accepted, although some of Rumford's contemporaries such as Laplace and John Dalton died unconvinced of the kinetic theory.
Rumford was also an inventor in the field of lighting, heating and cooking. He developed (around 1806) an oil lamp that gave six times more light than the existing Argand lamp, although the latter lamp, invented in 1780, was already a great improvement on the candle light in use before that. In order to make sure that developments on his lamps were really improvements, Rumford invented a photometer by which the yields of lamps could be assessed objectively.
The Rumford fireplace was briefly mentioned above. Further improvements were in the shape of the chimney and the fireplace itself. The Rumford fireplace radiated more heat for less fuel than the old fireplaces. The Rumford fireplace was a great success and is still on the market—mainly for the purpose of restoring old houses into their original state.
Another great success was his invention of an embryonic kitchen range, not unlike those used today, together with what came to be called a Rumford roaster. In the kitchen range up to twelve separate fireplaces could be built in and they were designed to heat special pots, pans, kettles, etc. all invented by Rumford. The Rumford roaster was designed for cooking meat that hitherto had been done on a spit over an open fire. Rumford also revolutionized cooking for armies in the field.
References and notes
- ↑ Thompson retired at the rank of full colonel, which awarded him half-pay for the rest of his life after only serving in the British army for sixteen months,
- ↑ In 1765 renamed to Concord. The city became state capital in 1808.
- ↑ James E. Bradley, "The Reprieve of a Loyalist: Count Rumford's Invitation Home." New England Quarterly 1974 47(3): 368-385. ISSN 0028-4866 in Jstor
- ↑ Antoine Lavoisier Traité élémentaire de chimie, 2 vols. Chez Cuchet, Paris (1789). Translated from the French by Robert Kerr, Elements of Chemistry, 4th edition. William Creech, Edinburgh: (1790) p. 175
- ↑ Later measurements refined this number to 4.184, and we are now so certain of the equivalence of work and heat that there is only one SI unit of energy, the joule. The calorie is defined as 4.184 joule.

