Carboxylic acid: Difference between revisions
imported>David E. Volk (picture of 3 carboxylic acids: acetic, alanine and benzoic) |
(A few minor edits, mostly removing dead red links) |
||
| (8 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
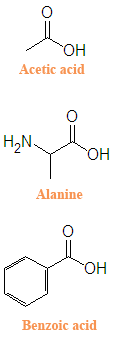
{{Image|Carboxylic_acids_acetic_acid%2C_alanine_and_benzoic_acid.png|right|300px|Structures of three carboxylic acids}} | |||
'''Carboxylic acids''' are important precursor chemicals both in the lab and in living organisms. The twenty common [[Amino acid|amino acids]] are a particularly important class of carboxylic acids. Carboxylic acids are generally tart in taste and are thus widely used in the food industry. Foods are often preserved with the addition of sodium benzoate, which is the conjugate base of benzoic | '''Carboxylic acids''' are important precursor chemicals both in the lab and in living organisms. The twenty common [[Amino acid|amino acids]] are a particularly important class of carboxylic acids. Carboxylic acids are generally tart in taste and are thus widely used in the food industry. Foods are often preserved with the addition of sodium benzoate, which is the conjugate base of benzoic acid. [[Acetic acid]] is a carboxylic acid that gives vinegar its bite. [[Formic acid]] has historically been used to preserve specimens of animal tissue. | ||
== | == Chemical properties == | ||
Carboxylic acids contain a carbon atom that is attached to one oxygen with a double bond, a | Carboxylic acids contain a carbon atom that is attached to one oxygen atom with a double bond, a hydroxyl group by a single bond, and one additional bond to an organic group (R–), which is often an [[alkane]] or [[alkene]] group. They are often written as R–C(=O)OH, R–COOH, or R–CO<sub>2</sub>H. Carboxylic acids are weak Brønsted-Lowry acids, which only partially dissociate in water to produce the hydronium ion [H<sub>3</sub>O]<sup>+</sup> and the conjugate base of the acid, [R–CO<sub>2</sub>]<sup>–</sup>. Although small, carboxylic acids are soluble in water; larger acids are increasingly insoluble in water but increasingly soluble in non-polar solvents. | ||
== | == Stability of the conjugate base == | ||
The negative charge of the conjugate base is shared between the two electronegative oxygen atoms, and this effect can be visualized as two different resonance structures. The strength of carboxylic acids is affected by the organic group to which it is attached. If the R group is an electron acceptor, it can stabilize the negative charge of the conjugate base either through inductive or resonance effects and therefore increase the acid strength. | |||
[[ | == Chemical reactions == | ||
[[Category: | Carboxylic acids can be formed by the hydrolysis of a corresponding [[ester]] or [[amide]]. Thus, ethyl acetate can be hydrolyzed to form acetic acid and ethanol. Conversely, the Fisher esterification reaction can be used to react a carboxylic acid with an alcohol to form an ester. Carboxylic acids can also be reduced to form aldehydes or further reduced to form primary alcohols. | ||
== Flavoring agents == | |||
Their tart taste makes many carboxylic acids useful as flavoring agents in the food industry. Citric acid is widely used in fruit punches and candy. | |||
==References== | |||
{{reflist}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 10:53, 25 July 2024
Carboxylic acids are important precursor chemicals both in the lab and in living organisms. The twenty common amino acids are a particularly important class of carboxylic acids. Carboxylic acids are generally tart in taste and are thus widely used in the food industry. Foods are often preserved with the addition of sodium benzoate, which is the conjugate base of benzoic acid. Acetic acid is a carboxylic acid that gives vinegar its bite. Formic acid has historically been used to preserve specimens of animal tissue.
Chemical properties
Carboxylic acids contain a carbon atom that is attached to one oxygen atom with a double bond, a hydroxyl group by a single bond, and one additional bond to an organic group (R–), which is often an alkane or alkene group. They are often written as R–C(=O)OH, R–COOH, or R–CO2H. Carboxylic acids are weak Brønsted-Lowry acids, which only partially dissociate in water to produce the hydronium ion [H3O]+ and the conjugate base of the acid, [R–CO2]–. Although small, carboxylic acids are soluble in water; larger acids are increasingly insoluble in water but increasingly soluble in non-polar solvents.
Stability of the conjugate base
The negative charge of the conjugate base is shared between the two electronegative oxygen atoms, and this effect can be visualized as two different resonance structures. The strength of carboxylic acids is affected by the organic group to which it is attached. If the R group is an electron acceptor, it can stabilize the negative charge of the conjugate base either through inductive or resonance effects and therefore increase the acid strength.
Chemical reactions
Carboxylic acids can be formed by the hydrolysis of a corresponding ester or amide. Thus, ethyl acetate can be hydrolyzed to form acetic acid and ethanol. Conversely, the Fisher esterification reaction can be used to react a carboxylic acid with an alcohol to form an ester. Carboxylic acids can also be reduced to form aldehydes or further reduced to form primary alcohols.
Flavoring agents
Their tart taste makes many carboxylic acids useful as flavoring agents in the food industry. Citric acid is widely used in fruit punches and candy.