Aripiprazole: Difference between revisions
imported>Tom Morris No edit summary |
mNo edit summary |
||
| (5 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
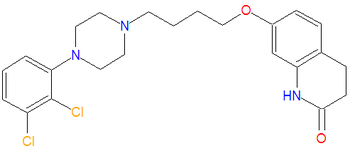
{{Chem infobox | |||
|align=right | |||
|image=[[Image:Aripiprazole.png|center|thumb|350px]] | |||
|width=350px | |||
|molname=Aripiprazole | |||
|synonyms= | |||
|molformula= C<sub>23</sub>H<sub>27</sub>Cl<sub>2</sub>N<sub>3</sub>O<sub>2</sub> | |||
|molmass= 448.38 | |||
|uses=[[antipsychotic agent]] | |||
|properties=medication | |||
|hazards=see side effects & drug interactions | |||
|iupac= 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydrocarbostyril | |||
|casnumber= | |||
}} | |||
In the [[United States]], the [[Food and Drug Administration]] has approved the use of aripiprazole for the treatment of [[schizophrenia]], [[bipolar disorder|bipolar I disorder]], [[major depressive disorder]], irritability associated with [[autistic disorder]] agitation associated with [[schizophrenia]] or mania from [[bipolar disorder]]. The trade name is Abilify™. Although not approved for the treatment of [[dementia]], aripiprazole has been studied in this setting. | |||
In [[medicine]], '''aripiprazole''' (pronunciation: ay ri pip' ray zole) is an atypical or second generation [[antipsychotic agent]] that "has both presynaptic [[dopamine]] autoreceptor agonistic activity and postsynaptic D<sub>2</sub> receptor antagonistic activity; structure given in first source; use associated with hyperglycemia."<ref>{{MeSH}}</ref> | |||
In the [[United States of America]], the [[Food and Drug Administration]] has approved the use of aripiprazole for the treatment of [[schizophrenia]], [[bipolar disorder|bipolar I disorder]], [[major depressive disorder]], irritability associated with [[autistic disorder]] agitation associated with [[schizophrenia]] or mania from [[bipolar disorder]]. It is also approved as an adjunct to antidepressants.<ref>{{citation | |||
| title = Beneficial acute antidepressant effects of aripiprazole as an adjunctive treatment or monotherapy in bipolar patients unresponsive to mood stabilizers: results from a 16-week open-label trial | |||
| date = December 2008 | volume = 9 | issue = 18 | pages = 3145-3149 | doi =10.1517/14656560802504490 | |||
| journal = Expert Opinion on Pharmacotherapy | |||
| author = Marianna Mazza, Maria Rosaria Squillacioti1, Riccardo Daniele Pecora, Luigi Janiri1 & Pietro Bria | |||
| url = http://informahealthcare.com/doi/abs/10.1517/14656560802504490}}</ref> The trade name is Abilify™. Although not approved for the treatment of [[dementia]], aripiprazole has been studied in this setting. | |||
==References== | ==References== | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 16:00, 12 July 2024
|
| |||||||
| Aripiprazole | |||||||
| |||||||
| Uses: | antipsychotic agent | ||||||
| Properties: | medication | ||||||
| Hazards: | see side effects & drug interactions | ||||||
| |||||||
In medicine, aripiprazole (pronunciation: ay ri pip' ray zole) is an atypical or second generation antipsychotic agent that "has both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity; structure given in first source; use associated with hyperglycemia."[1]
In the United States of America, the Food and Drug Administration has approved the use of aripiprazole for the treatment of schizophrenia, bipolar I disorder, major depressive disorder, irritability associated with autistic disorder agitation associated with schizophrenia or mania from bipolar disorder. It is also approved as an adjunct to antidepressants.[2] The trade name is Abilify™. Although not approved for the treatment of dementia, aripiprazole has been studied in this setting.
References
- ↑ Anonymous (2024), Aripiprazole (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ Marianna Mazza, Maria Rosaria Squillacioti1, Riccardo Daniele Pecora, Luigi Janiri1 & Pietro Bria (December 2008), "Beneficial acute antidepressant effects of aripiprazole as an adjunctive treatment or monotherapy in bipolar patients unresponsive to mood stabilizers: results from a 16-week open-label trial", Expert Opinion on Pharmacotherapy 9 (18): 3145-3149, DOI:10.1517/14656560802504490