Aminostatic hypothesis: Difference between revisions
imported>Gianna Maurer |
mNo edit summary |
||
| (47 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Image|Appetite.jpg|right|250px}} | {{Image|Appetite.jpg|right|250px}} | ||
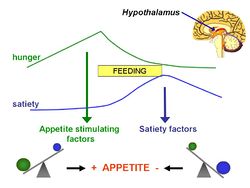
In 1956, Mellinkoff proposed ''' | In 1956, Mellinkoff proposed the '''aminostatic hypothesis''', stimulated by the observation that when normal individuals ingest [[protein]], [[appetite]] diminishes as the serum [[amino acid]] concentration rises and vice versa.<ref>Mellinkoff SM ''et al.'' (1956) Relationship between serum amino acid concentration and fluctuations in appetite ''J Appl Physiol'' 8:535-8 PMID 13295170</ref> He believed this was due to a satiety centre in the brain, sensitive to serum amino acids levels, that caused a suppression of hunger once the serum levels reached a certain point. | ||
== Experimental Evidence == | == Experimental Evidence == | ||
'''Mellinkoff's experiments''' | |||
In 1956, Mellinkoff first observed the relationship between protein intake and hunger levels in human subjects.<ref>Mellinkoff ''et al.'' (1956) Relationship between serum amino acid concentration and fluctuations in appetite. ''J App Physiol'' 8 :535– 88</ref> He conducted four sets of experiments, each investigating the effect of ingested or infused protein (i.e. increased serum amino acid concentrations) on appetite. In experiments 1 and 2, protein was ingested (exp. 1) or infused in 45 minutes (exp. 2) together with glucose. For experiments 3 and 4 protein alone, in the form of casein, was ingested (exp. 3) or infused in 45 minutes (exp. 4). Serum amino acid concentrations were measured for 4 hours at regular intervals and subjects were asked to determine their level of hunger using a scale from -1 (nauseated) to +4 (ravenous) for all four experiments. | |||
In 1956, | |||
In all experiments, appetite decreased as serum amino acid concentration increased and vice versa, illustrating a reciprocal relationship between appetite and serum amino acid concentrations. In experiments 1 and 2, appetite varied inversely with both serum glucose and amino acid concentrations while in experiments 3 and 4, where casein was administered alone, appetite was found to correlate inversely with serum amino acid but not glucose concentrations. This showed, contrary to popular belief that the decrease in appetite was not simply due to an increase in blood glucose, but that increased serum amino acid concentration had an independent effect on appetite and plays a role in the control of satiety. | |||
'''Increased serum amino acid concentrations and satiety''' | '''Increased serum amino acid concentrations and satiety''' | ||
... | Since Melinkoff’s experiments, research has aimed to clarify and validate the idea that appetite diminishes as serum amino acid concentrations increase. To investigate the effects of protein diets on satiety, several preloads with differing protein/nutrient concentrations are given to subjects, then subjects rate their satiety according to a set scale after determined time points.<ref name=Halton04>Halton ''et al.'' (2004) The effects of high protein diets on themogenesis, satiety and weight loss: a critical review. ''J Am Coll Nutr'' 23:373-85</ref> Poppitt ''et al.''<ref>Poppitt SD ''et al.'' (1998) Short term effects of macronutrient preloads on appetite and energy intake in lean women ''Physiol Behav'' 64:279–85</ref> found that subject were significantly less hungry after a high protein preload than preloads containing high levels of [[carbohydrate]]s or fats. Similarly Westerterp-Plantenga <ref>Westerterp-Plantenga MS ''et al.'' (1999) Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber ''Eur J Clin Nutr'' 53:495–502 </ref>found a significant increase in 24-hour satiety in subjects consuming a high-protein diet compared to a high-fat diet. Several other studies have also concluded that high-protein diets result in a higher levels of satiety, supporting the aminostatic hypothesis. <ref name=Halton04/> | ||
'''Increased serum amino acid concentrations and thermogenesis''' | '''Increased serum amino acid concentrations and thermogenesis''' | ||
... | The ingestion of high protein diets have been suggested to increase [[thermogenesis]], i.e. increase energy expenditure above baseline levels after consumption. <ref>Westerterp-Plantenga MS (2008) Protein intake and energy balance. ''Reg Peptides'' 149:67-9</ref> Johnston <ref>Johnston CS ''et al.'' (2002) Postprandial thermogenesis is increased 100% on a high protein, low fat diet versus a high carbohydrate low fat diet in healthy young women ''J Am Coll Nut'' 21:55–61</ref> found that diets with higher levels of protein have a greater effect on energy expenditure than diets with lower protein levels. A diet consisting of 15% protein had less thermic effect (-34kj/hour) than a diet with 30% protein. High protein diets have also been found to increase thermogenesis to a greater extent than high carbohydrate diets <ref> Luscombe ND ''et al.'' (2003) Effect of a high protein, energy restricted diet on weight loss and energy expenditure after weight stabilization in hyperinsulinemic subjects ''Int J Obes'' 27 :582–90</ref>. | ||
'''( | |||
- | |||
== Science behind the theory == | == Science behind the theory == | ||
{{Image|Aminostatic pathways.png|right|350px|Signalling Pathways.}}''' | |||
''' | ''' | ||
Experimental evidence supports the aminostatic hypothesis, but the complex homeostatic mechanisms between the peripheral organs and the central nervous system which cause this effect are not yet fully understood. Several different peptides released in response to dietary amino acids cause a decrease in orexigenic signalling and an increase in anorexigenic signalling by acting on one of two brain areas; the [[nucleus of the solitary tract]] (NTS) and the hypothalamic [[arcuate nucleus]].<ref name=Tome09>Tome D ''et al.'' (2009) Protein, amino acids, vagus nerve signalling and the brain ''Am J Clin Nutr'' 90:838-43</ref> The involvement of these brain areas has been confirmed through studies which have found increased activation of neurons in the NTS and increase activation of the melanocortin pathway in the acruate nucleus after ingestion of a high protein meal. <ref>Faipoux R ''et al.'' (2008) Proteins activate satiety-related neuronal pathways in the brainstem and hypothalamus of rats ''Am Soc Nutr'' 138:1172–8</ref> | |||
Experimental evidence supports the aminostatic hypothesis but the complex homeostatic mechanisms between the peripheral organs and the central nervous system which cause this effect are not yet fully understood. | |||
<br />The post-prandial signals act on specific brain areas through two different methods: | <br />The post-prandial signals act on specific brain areas through two different methods: | ||
<br />1) Indirectly though vagus-mediated pathways | <br />1) Indirectly though vagus-mediated pathways | ||
<br />2) Directly after their release into the peripheral blood | <br />2) Directly after their release into the peripheral blood <ref name=Tome09/> | ||
'''The indirect pathway''' | '''The indirect pathway''' | ||
<br />In the indirect pathway dietary protein and amino acids present in the small intestine act on chemoreceptors on the mucosal enteroendocrine cells causing the release of cholecystokinin (CCK). The CCK releasing cells | <br />In the indirect pathway, dietary protein and amino acids present in the [[small intestine]] act on chemoreceptors on the mucosal enteroendocrine cells causing the release of [[cholecystokinin]] (CCK). The CCK-releasing cells are very close to the vagal afferent fibres which express CCK type 1 receptors. Activation of the afferent vagus nerve by CCK is relayed to the nucleus of solitary tract, which mediates anorexigenic effects. <ref name=Tome09/> The anoretic effects have been confirmed by experiments involving the administration of a CCK antagonist which caused an increase in energy intake. <ref name=Lopez07>Lopez M ''et al.'' (2007)Peripheral tissue-brain interactions in the regulation of food intake ''Proc Nutr Soc'' 66:131–55</ref> This CCK-induced anorexia is thought to be a main mediator of satiety. | ||
'''The direct pathway''' | '''The direct pathway''' | ||
<br />The direct pathway involves peptide YY (PPY) which is secreted by L cells in the GI tract in response to dietary amino acids. | <br />The direct pathway involves [[peptide YY]] (PPY) which is secreted by L cells in the GI tract in response to dietary amino acids.<ref name=Lopez07/> PPY acts on the Y2 receptors of the [[neuropeptide Y]] neurons in the arcuate nucleus. This inhibits the secretion of neuropeptide Y and [[agouti-related protein]], potent orexigenic peptides, releasing the block on POMC neurons which allows them to release alpha-MSH to act on the MC4R receptors to cause anoretic effects.<ref name=Lopez07/> There is some controversy about the action of PPY, because in studies in rodents, primates and humans where it was administered peripherally it caused a decrease in food intake and a reduction in weight gain as expected but when it was given centrally it had the opposite effect and stimulated appetite. <ref name=Lopez07/> | ||
<br /><br />Other | <br /><br />'''Other factors thought to be involved'''<br />Other factors thought to be involved in satiety and reduced food intake due to a high protein diet are AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR). <ref name=Ropelle08>Ropelle ER ''et al.'' (2008) A central role for neuronal AMP-activated protein kinase and mammalian target of rapamycin in high protein diet induced weight loss ''Diabetes'' 57:594-605</ref> Studies found that a high protein diet caused suppression of AMPK phosphorylation and an activation of mTOR in the hypothalamus. It is thought that this is due to the actions of leucine which is the most abundant amino acid in most dietary proteins. It has been found to stimulate mTOR and cause the block in AMPK signalling ''in vivo'', however the mechanisms behind this are unknown <ref name=Ropelle08/> High protein diets enhanced with extra leucine were found to cause a lower food intake and an increased weight loss compared to diets with lower leucine content. <ref name=Ropelle08/> The involvement of mTOR in satiating pathways was confirmed by experiments which found that blocking mTOR reversed the anoretic effects of a high protein diet. <ref name=Ropelle08/> The activation of AMPK has been found to cause an increase in food intake and weigh gain. <ref name=Ropelle08/>The neurons which express AMPK and mTOR are found in the arcuate nucleus and it is suggested they are involved in PPY signalling.<ref name=Tome09/> | ||
<br /><br />The idea that | <br /><br />The idea that [[glucagon-like peptide 1]] (GLP-1) inhibits food intake through taste aversion was considered as it is released in response to amino acids in the gut and acts through the vagus afferent fibres. This theory was disproved after it was found that neurons expressing GLP-1 in the NTS were not activated during the satiety response.<ref name=Tome09/> Therefore the reduction in food intake after a high protein meal is not due to taste aversion. | ||
== Use as a method of weight loss == | == Use as a method of weight loss == | ||
''' | ''' | ||
Over the past few years, obesity has become a global epidemic. One third of American adults and approximately 17% of American children are obese ( | Over the past few years, obesity has become a global epidemic. One third of American adults and approximately 17% of American children are obese. <ref>Centers for Disease Control and Prevention (2011) U.S Obesity Trends. Available: [http://www.cdc.gov/obesity/data/trends.html] Last accessed 25th Oct 2011.</ref> In the UK, the statistics are not looking much brighter with 25% of adults and 10% of children showing signs of obesity. It has been proposed in the UK that 60% of men, 50% of women and 25% of children will become obese by 2050 if no preventative measures are taken.<ref>Department of Health (2011) Obesity. Available: [http://www.dh.gov.uk/en/Publichealth/Obesity/index.htm.] Last accessed 25th Oct 2011.</ref> | ||
The | Many hypotheses regarding various weight loss diets have been proposed, but what about the aminostatic hypothesis which suggests high protein intake? The science behind the theory certainly looks promising. It demonstrates induced levels of [[thermogenesis]] and increased [[satiety]], both of which could contribute to weight loss. | ||
High-protein diets are becoming increasingly popular all over the world, but are these diets just a fad? Protein intake normally consists of 10-15% from daily calorie allowance. However, a high protein diets consists of obtaining 30-40% of daily calories from protein alone. So, what does a high protein diet consist of and what are the benefits and limitations? Is there any evidence proving the success of this diet? | |||
The | The [[Dukan diet]] is most popular in France, where it was founded by Pierre Dukan. It is based on an allowance of one hundred allowed high protein foods controlled by four phases named attack, cruise, consolidation and stabilisation. This involves a strict regime of initially only eating 72 ‘allowed’ high protein foods (attack phase) for seven days which include beef, chicken and eggs. The next two stages slowly introduce vegetables (cruise phase) and other pleasurable foods (consolidation phase). Finally, other food groups are introduced in the stabilisation phase. <ref>Dukan P. (2011) The Dukan method: lose weight naturally. Available: [http://www.dukandiet.co.uk/en/336-dukan-coaching/the-dukan-method.html] Last accessed 6th Nov 2011. </ref> | ||
The [[Atkins diet]] is most popular in America but is slowly gaining recognition in other parts of the world, including the UK. Like the Dukan diet, it follows a strict regime of ‘allowed’ high protein foods. However, the Atkins diet is strictly no refined carbs. Dr Atkins believes many refined carbohydrate foods, such as pasta, also have high sugar levels. Limiting the amount of carbohydrates a person eats would force the body to burn off fat instead of carbs.<ref>Atkins International (2011) Thoughtful Approach. Powerful Science. Available: [http://uk.atkins.com/science] Last accessed 6th Nov 2011.</ref> Restricting refined carbohydrates, and therefore restricting refined sugars, would also diminish the ‘sugar rollercoaster’ that many people experience throughout the day. Thus, the individual would not crave sugary foods and would feel satisfied for longer. | |||
Many clinical trials have been carried out to assess the effects of high-protein diets. A meta-analysis of five random clinical trials (N=447) showed that dieters on high-protein diets lost more weight, after 6 months, than dieters on the common low fat diets. The mean difference was -3.3kg. <ref>Brehma BJ ''et al.'' (2008) Benefits of high-protein weight loss diets: enough evidence for practice? ''Curr Opin Endocrinol'' 15:416-21</ref> However, the same studies showed no difference in weight loss after 12 months of dieting. This suggests that high protein diets are only successful for short term weight loss. However, another study compared four different diets consisting of varied quantities of carbohydrate and protein in pre-menopausal obese women (N=311). After 12 months of dieting, women who followed the high protein diet showed greater weight loss (mean = -4.7kg) compared to women subjected to the other diets named Zone (mean= -1.6kg), LEARN (mean=-2.6kg) and Ornish (mean=-2.2kg), respectively <ref>Gardner CD ''et al.'' (2007) Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial ''JAMA'' 297: 969-77</ref>. Moreover, a study by Westerterp-Plantenga ''et al.'' (2006) <ref>Westerterp-Plantenga MS ''et al.'' (2006) Dietary protein, metabolism, and body-weight regulation: dose–response effects. ''Int J Obesity'' 30:16-23</ref> concluded that high protein diets are more successful when intake is ad libitum. This supports the evidence that protein has a satiating effect which makes it less likely for impulsive, random eating. | |||
However, there are some safety concerns with many diets. With high-protein diets, there is major concern over renal failure and bone loss. Although evidence is ambiguous regarding high protein diets damaging the kidneys, high-protein diets are best avoided in patients with underlying kidney problems. High-protein diets also propose a concern over bone health. For example, when meat is eaten, calcium is excreted from the bones to fulfil metabolic requirements. Reduced calcium levels in bones could potentially lead to [[osteoporosis]]. | |||
Most studies have concluded that high protein diets do help to lose weight in the short term. However, high protein foods need to be eaten with limiting carbohydrates and fats to gain the maximum benefits. Although this sounds promising, potential detrimental long-term effects such as renal failure and bone loss still need to be addressed. | |||
== '''Conclusion''' == | == '''Conclusion''' == | ||
Since Mellinkoff's experiment in 1956 much progress has been made into understanding the complex mechanisms that give rise to the aminostatic hypothesis but there are still details that are not yet fully understood. There is evidence that increased serum amino acid concentration and high protein diets promote satiety and increase thermogenesis. The effectiveness of protein diets on inducing satiety are very much determined by the amount of protein present in the diet, as high-protein diets have been shown to be more effective at reducing appetite than diets with medium levels of protein. If high- protein diets are to be used in the future, as a method of weight loss, more research into potential long term effects need to be addressed. | |||
==References== | ==References== | ||
{{reflist | 2}} | {{reflist | 2}}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 16:01, 9 July 2024
In 1956, Mellinkoff proposed the aminostatic hypothesis, stimulated by the observation that when normal individuals ingest protein, appetite diminishes as the serum amino acid concentration rises and vice versa.[1] He believed this was due to a satiety centre in the brain, sensitive to serum amino acids levels, that caused a suppression of hunger once the serum levels reached a certain point.
Experimental Evidence
Mellinkoff's experiments
In 1956, Mellinkoff first observed the relationship between protein intake and hunger levels in human subjects.[2] He conducted four sets of experiments, each investigating the effect of ingested or infused protein (i.e. increased serum amino acid concentrations) on appetite. In experiments 1 and 2, protein was ingested (exp. 1) or infused in 45 minutes (exp. 2) together with glucose. For experiments 3 and 4 protein alone, in the form of casein, was ingested (exp. 3) or infused in 45 minutes (exp. 4). Serum amino acid concentrations were measured for 4 hours at regular intervals and subjects were asked to determine their level of hunger using a scale from -1 (nauseated) to +4 (ravenous) for all four experiments.
In all experiments, appetite decreased as serum amino acid concentration increased and vice versa, illustrating a reciprocal relationship between appetite and serum amino acid concentrations. In experiments 1 and 2, appetite varied inversely with both serum glucose and amino acid concentrations while in experiments 3 and 4, where casein was administered alone, appetite was found to correlate inversely with serum amino acid but not glucose concentrations. This showed, contrary to popular belief that the decrease in appetite was not simply due to an increase in blood glucose, but that increased serum amino acid concentration had an independent effect on appetite and plays a role in the control of satiety.
Increased serum amino acid concentrations and satiety
Since Melinkoff’s experiments, research has aimed to clarify and validate the idea that appetite diminishes as serum amino acid concentrations increase. To investigate the effects of protein diets on satiety, several preloads with differing protein/nutrient concentrations are given to subjects, then subjects rate their satiety according to a set scale after determined time points.[3] Poppitt et al.[4] found that subject were significantly less hungry after a high protein preload than preloads containing high levels of carbohydrates or fats. Similarly Westerterp-Plantenga [5]found a significant increase in 24-hour satiety in subjects consuming a high-protein diet compared to a high-fat diet. Several other studies have also concluded that high-protein diets result in a higher levels of satiety, supporting the aminostatic hypothesis. [3]
Increased serum amino acid concentrations and thermogenesis
The ingestion of high protein diets have been suggested to increase thermogenesis, i.e. increase energy expenditure above baseline levels after consumption. [6] Johnston [7] found that diets with higher levels of protein have a greater effect on energy expenditure than diets with lower protein levels. A diet consisting of 15% protein had less thermic effect (-34kj/hour) than a diet with 30% protein. High protein diets have also been found to increase thermogenesis to a greater extent than high carbohydrate diets [8].
Science behind the theory
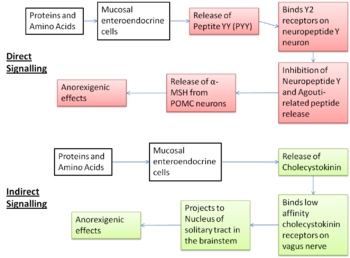
Experimental evidence supports the aminostatic hypothesis, but the complex homeostatic mechanisms between the peripheral organs and the central nervous system which cause this effect are not yet fully understood. Several different peptides released in response to dietary amino acids cause a decrease in orexigenic signalling and an increase in anorexigenic signalling by acting on one of two brain areas; the nucleus of the solitary tract (NTS) and the hypothalamic arcuate nucleus.[9] The involvement of these brain areas has been confirmed through studies which have found increased activation of neurons in the NTS and increase activation of the melanocortin pathway in the acruate nucleus after ingestion of a high protein meal. [10]
The post-prandial signals act on specific brain areas through two different methods:
1) Indirectly though vagus-mediated pathways
2) Directly after their release into the peripheral blood [9]
The indirect pathway
In the indirect pathway, dietary protein and amino acids present in the small intestine act on chemoreceptors on the mucosal enteroendocrine cells causing the release of cholecystokinin (CCK). The CCK-releasing cells are very close to the vagal afferent fibres which express CCK type 1 receptors. Activation of the afferent vagus nerve by CCK is relayed to the nucleus of solitary tract, which mediates anorexigenic effects. [9] The anoretic effects have been confirmed by experiments involving the administration of a CCK antagonist which caused an increase in energy intake. [11] This CCK-induced anorexia is thought to be a main mediator of satiety.
The direct pathway
The direct pathway involves peptide YY (PPY) which is secreted by L cells in the GI tract in response to dietary amino acids.[11] PPY acts on the Y2 receptors of the neuropeptide Y neurons in the arcuate nucleus. This inhibits the secretion of neuropeptide Y and agouti-related protein, potent orexigenic peptides, releasing the block on POMC neurons which allows them to release alpha-MSH to act on the MC4R receptors to cause anoretic effects.[11] There is some controversy about the action of PPY, because in studies in rodents, primates and humans where it was administered peripherally it caused a decrease in food intake and a reduction in weight gain as expected but when it was given centrally it had the opposite effect and stimulated appetite. [11]
Other factors thought to be involved
Other factors thought to be involved in satiety and reduced food intake due to a high protein diet are AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR). [12] Studies found that a high protein diet caused suppression of AMPK phosphorylation and an activation of mTOR in the hypothalamus. It is thought that this is due to the actions of leucine which is the most abundant amino acid in most dietary proteins. It has been found to stimulate mTOR and cause the block in AMPK signalling in vivo, however the mechanisms behind this are unknown [12] High protein diets enhanced with extra leucine were found to cause a lower food intake and an increased weight loss compared to diets with lower leucine content. [12] The involvement of mTOR in satiating pathways was confirmed by experiments which found that blocking mTOR reversed the anoretic effects of a high protein diet. [12] The activation of AMPK has been found to cause an increase in food intake and weigh gain. [12]The neurons which express AMPK and mTOR are found in the arcuate nucleus and it is suggested they are involved in PPY signalling.[9]
The idea that glucagon-like peptide 1 (GLP-1) inhibits food intake through taste aversion was considered as it is released in response to amino acids in the gut and acts through the vagus afferent fibres. This theory was disproved after it was found that neurons expressing GLP-1 in the NTS were not activated during the satiety response.[9] Therefore the reduction in food intake after a high protein meal is not due to taste aversion.
Use as a method of weight loss
Over the past few years, obesity has become a global epidemic. One third of American adults and approximately 17% of American children are obese. [13] In the UK, the statistics are not looking much brighter with 25% of adults and 10% of children showing signs of obesity. It has been proposed in the UK that 60% of men, 50% of women and 25% of children will become obese by 2050 if no preventative measures are taken.[14]
Many hypotheses regarding various weight loss diets have been proposed, but what about the aminostatic hypothesis which suggests high protein intake? The science behind the theory certainly looks promising. It demonstrates induced levels of thermogenesis and increased satiety, both of which could contribute to weight loss. High-protein diets are becoming increasingly popular all over the world, but are these diets just a fad? Protein intake normally consists of 10-15% from daily calorie allowance. However, a high protein diets consists of obtaining 30-40% of daily calories from protein alone. So, what does a high protein diet consist of and what are the benefits and limitations? Is there any evidence proving the success of this diet?
The Dukan diet is most popular in France, where it was founded by Pierre Dukan. It is based on an allowance of one hundred allowed high protein foods controlled by four phases named attack, cruise, consolidation and stabilisation. This involves a strict regime of initially only eating 72 ‘allowed’ high protein foods (attack phase) for seven days which include beef, chicken and eggs. The next two stages slowly introduce vegetables (cruise phase) and other pleasurable foods (consolidation phase). Finally, other food groups are introduced in the stabilisation phase. [15]
The Atkins diet is most popular in America but is slowly gaining recognition in other parts of the world, including the UK. Like the Dukan diet, it follows a strict regime of ‘allowed’ high protein foods. However, the Atkins diet is strictly no refined carbs. Dr Atkins believes many refined carbohydrate foods, such as pasta, also have high sugar levels. Limiting the amount of carbohydrates a person eats would force the body to burn off fat instead of carbs.[16] Restricting refined carbohydrates, and therefore restricting refined sugars, would also diminish the ‘sugar rollercoaster’ that many people experience throughout the day. Thus, the individual would not crave sugary foods and would feel satisfied for longer.
Many clinical trials have been carried out to assess the effects of high-protein diets. A meta-analysis of five random clinical trials (N=447) showed that dieters on high-protein diets lost more weight, after 6 months, than dieters on the common low fat diets. The mean difference was -3.3kg. [17] However, the same studies showed no difference in weight loss after 12 months of dieting. This suggests that high protein diets are only successful for short term weight loss. However, another study compared four different diets consisting of varied quantities of carbohydrate and protein in pre-menopausal obese women (N=311). After 12 months of dieting, women who followed the high protein diet showed greater weight loss (mean = -4.7kg) compared to women subjected to the other diets named Zone (mean= -1.6kg), LEARN (mean=-2.6kg) and Ornish (mean=-2.2kg), respectively [18]. Moreover, a study by Westerterp-Plantenga et al. (2006) [19] concluded that high protein diets are more successful when intake is ad libitum. This supports the evidence that protein has a satiating effect which makes it less likely for impulsive, random eating.
However, there are some safety concerns with many diets. With high-protein diets, there is major concern over renal failure and bone loss. Although evidence is ambiguous regarding high protein diets damaging the kidneys, high-protein diets are best avoided in patients with underlying kidney problems. High-protein diets also propose a concern over bone health. For example, when meat is eaten, calcium is excreted from the bones to fulfil metabolic requirements. Reduced calcium levels in bones could potentially lead to osteoporosis.
Most studies have concluded that high protein diets do help to lose weight in the short term. However, high protein foods need to be eaten with limiting carbohydrates and fats to gain the maximum benefits. Although this sounds promising, potential detrimental long-term effects such as renal failure and bone loss still need to be addressed.
Conclusion
Since Mellinkoff's experiment in 1956 much progress has been made into understanding the complex mechanisms that give rise to the aminostatic hypothesis but there are still details that are not yet fully understood. There is evidence that increased serum amino acid concentration and high protein diets promote satiety and increase thermogenesis. The effectiveness of protein diets on inducing satiety are very much determined by the amount of protein present in the diet, as high-protein diets have been shown to be more effective at reducing appetite than diets with medium levels of protein. If high- protein diets are to be used in the future, as a method of weight loss, more research into potential long term effects need to be addressed.
References
- ↑ Mellinkoff SM et al. (1956) Relationship between serum amino acid concentration and fluctuations in appetite J Appl Physiol 8:535-8 PMID 13295170
- ↑ Mellinkoff et al. (1956) Relationship between serum amino acid concentration and fluctuations in appetite. J App Physiol 8 :535– 88
- ↑ 3.0 3.1 Halton et al. (2004) The effects of high protein diets on themogenesis, satiety and weight loss: a critical review. J Am Coll Nutr 23:373-85
- ↑ Poppitt SD et al. (1998) Short term effects of macronutrient preloads on appetite and energy intake in lean women Physiol Behav 64:279–85
- ↑ Westerterp-Plantenga MS et al. (1999) Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber Eur J Clin Nutr 53:495–502
- ↑ Westerterp-Plantenga MS (2008) Protein intake and energy balance. Reg Peptides 149:67-9
- ↑ Johnston CS et al. (2002) Postprandial thermogenesis is increased 100% on a high protein, low fat diet versus a high carbohydrate low fat diet in healthy young women J Am Coll Nut 21:55–61
- ↑ Luscombe ND et al. (2003) Effect of a high protein, energy restricted diet on weight loss and energy expenditure after weight stabilization in hyperinsulinemic subjects Int J Obes 27 :582–90
- ↑ 9.0 9.1 9.2 9.3 9.4 Tome D et al. (2009) Protein, amino acids, vagus nerve signalling and the brain Am J Clin Nutr 90:838-43
- ↑ Faipoux R et al. (2008) Proteins activate satiety-related neuronal pathways in the brainstem and hypothalamus of rats Am Soc Nutr 138:1172–8
- ↑ 11.0 11.1 11.2 11.3 Lopez M et al. (2007)Peripheral tissue-brain interactions in the regulation of food intake Proc Nutr Soc 66:131–55
- ↑ 12.0 12.1 12.2 12.3 12.4 Ropelle ER et al. (2008) A central role for neuronal AMP-activated protein kinase and mammalian target of rapamycin in high protein diet induced weight loss Diabetes 57:594-605
- ↑ Centers for Disease Control and Prevention (2011) U.S Obesity Trends. Available: [1] Last accessed 25th Oct 2011.
- ↑ Department of Health (2011) Obesity. Available: [2] Last accessed 25th Oct 2011.
- ↑ Dukan P. (2011) The Dukan method: lose weight naturally. Available: [3] Last accessed 6th Nov 2011.
- ↑ Atkins International (2011) Thoughtful Approach. Powerful Science. Available: [4] Last accessed 6th Nov 2011.
- ↑ Brehma BJ et al. (2008) Benefits of high-protein weight loss diets: enough evidence for practice? Curr Opin Endocrinol 15:416-21
- ↑ Gardner CD et al. (2007) Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial JAMA 297: 969-77
- ↑ Westerterp-Plantenga MS et al. (2006) Dietary protein, metabolism, and body-weight regulation: dose–response effects. Int J Obesity 30:16-23