User:John R. Brews/Sandbox: Difference between revisions
imported>John R. Brews |
imported>John R. Brews |
||
| Line 4: | Line 4: | ||
For ferromagnetic materials, the self-interaction of the atoms tends to align them even when no external magnetic field is present. As a result, ferromagnetic materials create a net magnetic field (and magnetic flux density) in the space surrounding the material, and can form permanent magnets at temperatures below the [[Curie temperature]] of the material. At higher temperatures, the aligning interaction is inadequate to overcome the randomness introduced by thermal motions, and the material becomes paramagnetic. | For ferromagnetic materials, the self-interaction of the atoms tends to align them even when no external magnetic field is present. As a result, ferromagnetic materials create a net magnetic field (and magnetic flux density) in the space surrounding the material, and can form permanent magnets at temperatures below the [[Curie temperature]] of the material. At higher temperatures, the aligning interaction is inadequate to overcome the randomness introduced by thermal motions, and the material becomes paramagnetic. | ||
The basis for cooperation between atomic magnetic moments is that electrons obey the [[Pauli exclusion principle]] that no two can occupy the same quantum state. That means configurations with aligned spins are energetically favored over misaligned spins by an ''exchange interaction'', favoring magnetization. The same idea underlies [[Hund's rules]] for atoms, namely, other things equal, electrons in atoms populate states to maximize their total spin. | The basis for cooperation between atomic magnetic moments is that electrons obey the [[Pauli exclusion principle]] that no two can occupy the same quantum state. That means configurations with aligned spins are energetically favored over misaligned spins by an ''exchange interaction'', favoring magnetization. The same idea underlies [[Hund's rules]] for atoms, namely, other things equal, electrons in atoms populate states to maximize their total spin. Not addressed here is the unanswered question: why do ferromagnetic materials profit from this effect more than other materials.<ref name=exchange> | ||
Revision as of 16:02, 15 December 2010
Ferromagnetism
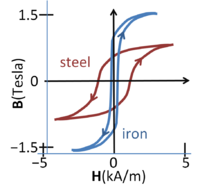
Add image caption here.Magnetic flux density vs. magnetic field in steel and iron; the curve depends upon the direction of traversal, the phenomenon of hysteresis
For ferromagnetic materials, the self-interaction of the atoms tends to align them even when no external magnetic field is present. As a result, ferromagnetic materials create a net magnetic field (and magnetic flux density) in the space surrounding the material, and can form permanent magnets at temperatures below the Curie temperature of the material. At higher temperatures, the aligning interaction is inadequate to overcome the randomness introduced by thermal motions, and the material becomes paramagnetic.
The basis for cooperation between atomic magnetic moments is that electrons obey the Pauli exclusion principle that no two can occupy the same quantum state. That means configurations with aligned spins are energetically favored over misaligned spins by an exchange interaction, favoring magnetization. The same idea underlies Hund's rules for atoms, namely, other things equal, electrons in atoms populate states to maximize their total spin. Not addressed here is the unanswered question: why do ferromagnetic materials profit from this effect more than other materials.[1]
The figure shows magnetization curves for two different ferromagnetic materials. The curves exhibit hysteresis, that is, the curve is history dependent and, in particular, depends upon the direction in which the magnetic field increases. This complex behavior indicates that magnetization in such materials is not an equilibrium process. Larger samples break up into magnetic domains or sub-regions of different magnetization directions separated by domain walls.[2] The magnetization curve is affected (in part) by the change in size of the various domains as they reluctantly adapt to changes in the external field.[3]
Today it is still impossible to predict from first principles that iron is ferromagnetic.[4] However, some guidance can be obtained as to which metals are candidates, and which are not, based upon estimates of how exchange energy varies with atomic radii and spacing. "The theory of magnetism in solids is one of the central challenges in condensed matter physics, intrinsically involving many-body correlations, long range order and phase transitions..."[5]
- ↑ For more about the exchange interaction, see LD Landau and EM Lifshitz (1960). “Chapter V: Ferromagnetism”, Electrodynamics of continuous media. Pergamon Press, pp. 146 ff. , and for some simple examples Assa Auerbach (1999). “Chapter 2: Spin exchange”, Interacting electrons and quantum magnetism. Springer, pp. 11 ff. ISBN 0387942866. .
- ↑ F Fiorillo, C Appino and M Pasquale (2005). “§1.3 Energy in a magnetic system. Domain walls and domain structures”, Isaak D. Mayergoyz, Giorgio Bertotti, editors: The Science of Hysteresis, volume III. Elsevier Academic Press, pp. 29 ff. ISBN 0123694337.
- ↑ Same reference as previously, but p. 169:J Kübler (2009). “§4.1.1 Stoner theory”, Theory of itinerant electron magnetism, Revised ed. Oxford, pp. 169 ff. ISBN 0199559023.
- ↑ Bernard Dennis Cullity, Chad D. Graham (2009). “Chapter 4: Ferromagnetism”, Introduction to magnetic materials, 2nd ed. Wiley-IEEE, p. 131. ISBN 0471477419.
- ↑ Richard M Martin (2004). Electronic structure: basic theory and practical methods. Cambridge University Press, p. 24. ISBN 0521782856.