Torcetrapib: Difference between revisions
imported>David E. Volk m (refs) |
imported>Howard C. Berkowitz No edit summary |
||
| Line 14: | Line 14: | ||
}} | }} | ||
Torcetrapib was a drug in development to be used to modulate cholesterol levels by its inhibition of [[cholesteryl ester transferase protein]] (CETP). Pfizer | Torcetrapib was a drug in development to be used to modulate cholesterol levels by its inhibition of [[cholesteryl ester transferase protein]] (CETP). | ||
Due to safety concerns, Pfizer announced its withdrawal of torcetrapib from development<ref> url=http://www.pfizer.com/pfizer/download/investors/financial/8k_1202_06.pdf</ref> Although torcetrapib quickly raised HDL [[cholesterol]] in the ILLUMINATE study, patients receiving both torcetrapib and [[atorvastatin]] has increased incidence of adverse cardiac events and death compared to the control group only receiving atorvastatin. Similar medications include [[anacetrapib]] and [[dalcetrapib]]. | |||
== Chemistry == | == Chemistry == | ||
Revision as of 14:03, 26 November 2010
|
| |||||||
| torcetrapib | |||||||
| |||||||
| Uses: | cardiac medication | ||||||
| Properties: | |||||||
| Hazards: | increased death and cardiac events | ||||||
| |||||||
Torcetrapib was a drug in development to be used to modulate cholesterol levels by its inhibition of cholesteryl ester transferase protein (CETP).
Due to safety concerns, Pfizer announced its withdrawal of torcetrapib from development[1] Although torcetrapib quickly raised HDL cholesterol in the ILLUMINATE study, patients receiving both torcetrapib and atorvastatin has increased incidence of adverse cardiac events and death compared to the control group only receiving atorvastatin. Similar medications include anacetrapib and dalcetrapib.
Chemistry
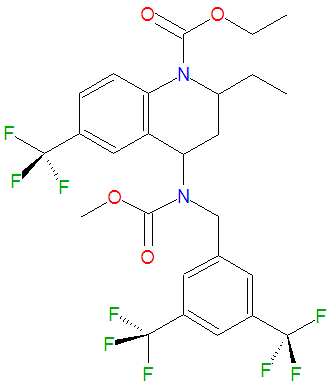
The chemical name of torcetrapib is (2R,4S)-4-[(3,5-Bis-trifluoromethylbenzyl)methoxycarbonylamino]-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester. The drug has a formula weight of 600.47 g/mol and is registered under the CAS number 262352-17-0.