Phosgene: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk m (tweak the stub) |
imported>David E. Volk No edit summary |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
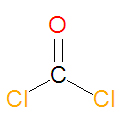
'''Phosgene''', the [[acid chloride]] of [[carbonic acid]], is an industrial chemical that was used as a [[chemical weapon]] during [[World War I]]. It is a [[choking gas]] that reacts with water to produce carbon dioxide and hydrogen chloride gas, which is corrosive. Exposure can lead to [[pulmonary edema]] and [[chemical pneumonitis]]. Phosgene has many different names, including carbon oxychloride, chloroformyl chloride, carbonyl chloride, carbonic dichloride, CG (military) and carbonyl dichloride. | |||
== Decomposition == | |||
Like its parent compound carbonic acid, phosgene is very reactive and decomposes in the presence of moisture to form [[carbon dioxide]] (CO2) and [[hydrochloric acid]] (HCl). The oxygen atom of water molecules act as [[nucleophile|nucleophiles]] and attack the central carbon atom. | |||
[[Image:Decomposition of phosgene to hydrochloric acid and carbon dioxide.jpg|left|thumb|600px|{{#ifexist:Template:Decomposition of phosgene to hydrochloric acid and carbon dioxide.jpg/credit|{{Decomposition of phosgene to hydrochloric acid and carbon dioxide.jpg/credit}}<br/>|}}Add image caption here.]] | |||
{{Chem infobox | {{Chem infobox | ||
| Line 15: | Line 23: | ||
|casnumber= 75-44-5 | |casnumber= 75-44-5 | ||
}} | }} | ||
Revision as of 16:51, 27 August 2008
Phosgene, the acid chloride of carbonic acid, is an industrial chemical that was used as a chemical weapon during World War I. It is a choking gas that reacts with water to produce carbon dioxide and hydrogen chloride gas, which is corrosive. Exposure can lead to pulmonary edema and chemical pneumonitis. Phosgene has many different names, including carbon oxychloride, chloroformyl chloride, carbonyl chloride, carbonic dichloride, CG (military) and carbonyl dichloride.
Decomposition
Like its parent compound carbonic acid, phosgene is very reactive and decomposes in the presence of moisture to form carbon dioxide (CO2) and hydrochloric acid (HCl). The oxygen atom of water molecules act as nucleophiles and attack the central carbon atom.
|
| |||||||
| phosgene | |||||||
| |||||||
| Uses: | chemical weapon | ||||||
| Properties: | corrosive | ||||||
| Hazards: | corrosive, produces chlorine gas | ||||||
| |||||||