Chloramphenicol: Difference between revisions
imported>David E. Volk mNo edit summary |
imported>Milton Beychok (Undo revision 100471687 by David E. Volk (Talk) Undid inadvertant deletion by David Volk) |
||
| Line 16: | Line 16: | ||
'''Chloramphenicol''' was the first broad-spectrum [[antibiotic]] discovered, in 1947, from ''[[Streptomyces venequelae]]'' cultures. Although it is active against a wide variety of organisms including [[tetracycline]]-resistant [[vibrio]]s, toxicity and safety concerns, such as bone marrow damage and anemia, typically limits its use to only the treatment of very serious infections, such as cholera and typhoid fever. The antibiotic works by binding to bacterial ribosome 50S subunits and inhibiting bacterial protein synthesis. | '''Chloramphenicol''' was the first broad-spectrum [[antibiotic]] discovered, in 1947, from ''[[Streptomyces venequelae]]'' cultures. Although it is active against a wide variety of organisms including [[tetracycline]]-resistant [[vibrio]]s, toxicity and safety concerns, such as bone marrow damage and anemia, typically limits its use to only the treatment of very serious infections, such as cholera and typhoid fever. The antibiotic works by binding to bacterial ribosome 50S subunits and inhibiting bacterial protein synthesis. | ||
== Chemistry == | |||
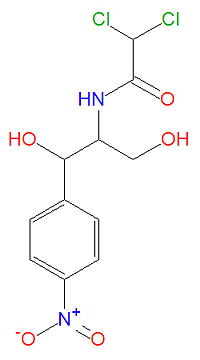
Chloroamphenicol, or 2,2-dichloro-N-[1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide, has molecular formula C<sub>11</sub>H<sub>12</sub>Cl<sub>2</sub>N<sub>2</sub>O<sub>5</sub> and molecular mass 323.1294 g/mol. The drug is referred to by several names, including CAF, CAM, CAP, chloramphenicole, chloramfenikol, chloroamphenicol, cloroamfenicolo, CPh, D-Chloramphenicol. | |||
== References == | |||
* {{DailyMed}} | |||
* {{MedMaster}} | |||
* {{DrugBank}} | |||
Revision as of 11:38, 6 April 2009
|
| |||||||
| chloramphenicol | |||||||
| |||||||
| Uses: | antibiotic | ||||||
| Properties: | |||||||
| Hazards: | toxicity | ||||||
| |||||||
Chloramphenicol was the first broad-spectrum antibiotic discovered, in 1947, from Streptomyces venequelae cultures. Although it is active against a wide variety of organisms including tetracycline-resistant vibrios, toxicity and safety concerns, such as bone marrow damage and anemia, typically limits its use to only the treatment of very serious infections, such as cholera and typhoid fever. The antibiotic works by binding to bacterial ribosome 50S subunits and inhibiting bacterial protein synthesis.
Chemistry
Chloroamphenicol, or 2,2-dichloro-N-[1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide, has molecular formula C11H12Cl2N2O5 and molecular mass 323.1294 g/mol. The drug is referred to by several names, including CAF, CAM, CAP, chloramphenicole, chloramfenikol, chloroamphenicol, cloroamfenicolo, CPh, D-Chloramphenicol.
References
- Chloramphenicol - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank