Sulfuric acid: Difference between revisions
imported>David E. Volk (typos) |
imported>Meg Taylor (update) |
||
| (9 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | |||
== | {{Chem infobox | ||
|align=right | |||
|image=[[Image:Sulfuric acid DEVolk.jpg|center|thumb|200px]] | |||
|width=200px | |||
|molname=sulfuric acid | |||
|synonyms= sulphuric acid and others | |||
|molformula= H<sub>2</sub>SO<sub>4</sub> | |||
|molmass= 98.08 | |||
|uses=acid, dehydration, reduction | |||
|properties=strong acid | |||
|hazards= Toxic, Corrosive | |||
|iupac= sulfuric acid | |||
|casnumber=7664-93-9 | |||
}} | |||
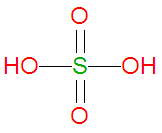
Because it has a high boiling point ( | '''Sulfuric acid''', also spelled '''sulphuric acid''', is a [[Strong acid|strong]], corrosive [[acid]] and [[oxidizing agent]] having the [[chemical formula]] H<sub>2</sub>SO<sub>4</sub>. It is the diprotic acid of the [[sulfate]] [[anion]] SO<sub>4</sub><sup><small>-2</small></sup>. At room temperature and pressure, it is a clear, colorless, rather [[Viscosity|viscous]] [[liquid]]. Sulfuric acid is one of the most important chemicals in the chemical industry. '''Personal protective gear should be worn when using sulfuric acid'''. | ||
== Synonyms == | |||
Sulfuric acid is also called oil of vitriol, mattling acid, vitriol, battery acid, dipping acid, electrolyte acid, vitriol brown oil, sulphuric acid, Babcock acid and sulphuric acid. | |||
== Properties and uses of sulfuric acid == | |||
Sulfuric acid is a [[strong acid]], an oxidizing agent and a dehydrating agent. Two [[hydrogen]] ions can dissociate from H<sub>2</sub>SO<sub>4</sub>. In an aqueous (water) [[Solution (chemistry)|solution]], the first hydrogen dissociates completely (100%) to form the ''bisulfate'' [[anion]] HSO<sub>4</sub><sup>-</sup>. Since this dissociation is complete, sulfuric acid is considered a strong acid. HSO<sub>4</sub><sup>-</sup> is a medium strength acid from which the second hydrogen dissociates to form the ''[[sulfate]]'' anion SO<sub>4</sub><sup><small>-2</small></sup>. | |||
: H<sub>2</sub>SO<sub>4</sub> + H<sub>2</sub>O → H<sub>3</sub>O<sup>+</sup> + HSO<sub>4</sub><sup>−</sup> K<sub>1</sub> = 2.4 x 10<sup>6</sup> (strong acid) | |||
: HSO<sub>4</sub><sup>−</sup> + H<sub>2</sub>0 → H<sub>3</sub>O<sup>+</sup> + SO<sub>4</sub><sup>2−</sup> K<sub>2</sub> = 1.0 x 10<sup>-2</sup> <ref>[http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/acidity.htm Ionization Constants of Inorganic Acids]</ref> | |||
K<sub>1</sub> and K<sub>2</sub> are the [[acid dissociation constant]]s. | |||
Sulfuric acid is used to make many soluble phophates for fertilizers, ammonium sulfate and many other chemicals, including drugs. Newly made steel is cleaned with sulfuric acid to remove rust before the steel is coated with a protective layer of zinc, tin or enamel. It is also used in lead sulfate batteries. Many explosives are manufactured using sulfuric acid. | |||
Because it has a high boiling point (33°C), it can be used to make other more volatile acids using the appropriate acid salt. [[Nitric acid]] can be made by reacting sulfuric acid with [[sodium nitrate]], NaNO<sub>3</sub>. Distilling the resultant nitric acid ([[Boiling point|BP]]=86°C) drives the [[Chemical reaction|reaction]] towards completion. | |||
: NaNO<sub>3</sub> + H<sub>2</sub>SO<sub>4</sub> → HNO<sub>3</sub> + NaHSO<sub>4</sub> | : NaNO<sub>3</sub> + H<sub>2</sub>SO<sub>4</sub> → HNO<sub>3</sub> + NaHSO<sub>4</sub> | ||
Contact of water and sulfuric acid is [[exothermic]]. When handling them, '''always add acid to water''', not the reverse or the resulting boiling can spray hot acid. | |||
The explosive [[nitroglycerin]] ([[glyceryl trinitrate]]), is made by reacting [[glycerine]] and nitric acid in the presence of sulfuric acid. '''This reaction is very dangerous, do not attempt.''' | The explosive [[nitroglycerin]] ([[glyceryl trinitrate]]), is made by reacting [[glycerine]] and nitric acid in the presence of sulfuric acid. '''This reaction is very dangerous, do not attempt.''' | ||
| Line 14: | Line 43: | ||
: C<sub>3</sub>H<sub>5</sub>(OH)<sub>3</sub> + 3HNO<sub>3</sub> + (H<sub>2</sub>SO<sub>4</sub> catalyst)→ C<sub>3</sub>H<sub>5</sub>(NO<sub>3</sub>)<sub>3</sub> + 3H<sub>2</sub>O. | : C<sub>3</sub>H<sub>5</sub>(OH)<sub>3</sub> + 3HNO<sub>3</sub> + (H<sub>2</sub>SO<sub>4</sub> catalyst)→ C<sub>3</sub>H<sub>5</sub>(NO<sub>3</sub>)<sub>3</sub> + 3H<sub>2</sub>O. | ||
Sulfuric acid can be used as a drying agent for | Sulfuric acid can be used as a drying agent for gases that do not react with sulfuric acid by bubbling the gas through sulfuric acid. | ||
== | == Synthesis of sulfuric acid == | ||
Sulfuric acid is made be reacting [[sulfur trioxide]] with water in an exothermic reaction. | Sulfuric acid is made be reacting [[sulfur trioxide]] with water in an exothermic reaction. | ||
| Line 29: | Line 58: | ||
:: 2) 2NOHSO<sub>4</sub> + H<sub>2</sub>O → 2H<sub>2</sub>SO<sub>4</sub> + NO + NO<sub>2</sub> | :: 2) 2NOHSO<sub>4</sub> + H<sub>2</sub>O → 2H<sub>2</sub>SO<sub>4</sub> + NO + NO<sub>2</sub> | ||
== | == Soluble and insoluble sulfate salts == | ||
The soluble salts of sulfate include [[sodium sulfate]] (Na<sub>2</sub>SO<sub>4</sub>) | The soluble salts of sulfate include [[sodium sulfate]] (Na<sub>2</sub>SO<sub>4</sub>)•10H<sub>2</sub>O, [[ammonium sulfate]] (NH<sub>4</sub>)<sub>2</sub>SO<sub>4</sub>, [[magnesium sulfate]] (Epsom salt, MgSO<sub>4</sub>•7H<sub>2</sub>O, [[copper sulfate]] ([[blue vitriol]], CuSO<sub>4</sub>•5H<sub>2</sub>O), iron sulfate (FeSO<sub>4</sub>•7H<sub>2</sub>O), [[zinc sulfate]] (ZnSO<sub>4</sub>•7H<sub>2</sub>O), [[potassium aluminium sulfate]] (alum, KAl(SO<sub>4</sub>)<sub>2</sub>•12H<sub>2</sub>O), [[ammonium aluminium sulfate]] (ammonium alum, NH<sub>4</sub>Al(SO<sub>4</sub>)<sub>2</sub>•12H<sub>2</sub>O), and chrome alum (KCr(SO<sub>4</sub>)<sub>2</sub>•12H<sub>2</sub>O). | ||
[[Barium sulfate]] (barite) is the least soluble sulfate salt and its white | [[Barium sulfate]] (barite) is the least soluble sulfate salt and its white precipitate is used as a test for sulfate [[anions]]. Other sulfates with diminished solubility include [[lead sulfate]] (PbSO<sub>4</sub>), [[strontium sulfate]] (SrSO4) and [[calcium sulfate]] ([[gypsum]], CaSO<sub>4</sub>•2H<sub>2</sub>O. | ||
Revision as of 05:47, 12 September 2013
|
| |||||||
| sulfuric acid | |||||||
| |||||||
| Uses: | acid, dehydration, reduction | ||||||
| Properties: | strong acid | ||||||
| Hazards: | Toxic, Corrosive | ||||||
| |||||||
Sulfuric acid, also spelled sulphuric acid, is a strong, corrosive acid and oxidizing agent having the chemical formula H2SO4. It is the diprotic acid of the sulfate anion SO4-2. At room temperature and pressure, it is a clear, colorless, rather viscous liquid. Sulfuric acid is one of the most important chemicals in the chemical industry. Personal protective gear should be worn when using sulfuric acid.
Synonyms
Sulfuric acid is also called oil of vitriol, mattling acid, vitriol, battery acid, dipping acid, electrolyte acid, vitriol brown oil, sulphuric acid, Babcock acid and sulphuric acid.
Properties and uses of sulfuric acid
Sulfuric acid is a strong acid, an oxidizing agent and a dehydrating agent. Two hydrogen ions can dissociate from H2SO4. In an aqueous (water) solution, the first hydrogen dissociates completely (100%) to form the bisulfate anion HSO4-. Since this dissociation is complete, sulfuric acid is considered a strong acid. HSO4- is a medium strength acid from which the second hydrogen dissociates to form the sulfate anion SO4-2.
- H2SO4 + H2O → H3O+ + HSO4− K1 = 2.4 x 106 (strong acid)
- HSO4− + H20 → H3O+ + SO42− K2 = 1.0 x 10-2 [1]
K1 and K2 are the acid dissociation constants.
Sulfuric acid is used to make many soluble phophates for fertilizers, ammonium sulfate and many other chemicals, including drugs. Newly made steel is cleaned with sulfuric acid to remove rust before the steel is coated with a protective layer of zinc, tin or enamel. It is also used in lead sulfate batteries. Many explosives are manufactured using sulfuric acid.
Because it has a high boiling point (33°C), it can be used to make other more volatile acids using the appropriate acid salt. Nitric acid can be made by reacting sulfuric acid with sodium nitrate, NaNO3. Distilling the resultant nitric acid (BP=86°C) drives the reaction towards completion.
- NaNO3 + H2SO4 → HNO3 + NaHSO4
Contact of water and sulfuric acid is exothermic. When handling them, always add acid to water, not the reverse or the resulting boiling can spray hot acid.
The explosive nitroglycerin (glyceryl trinitrate), is made by reacting glycerine and nitric acid in the presence of sulfuric acid. This reaction is very dangerous, do not attempt.
- C3H5(OH)3 + 3HNO3 + (H2SO4 catalyst)→ C3H5(NO3)3 + 3H2O.
Sulfuric acid can be used as a drying agent for gases that do not react with sulfuric acid by bubbling the gas through sulfuric acid.
Synthesis of sulfuric acid
Sulfuric acid is made be reacting sulfur trioxide with water in an exothermic reaction.
- SO3(g) + H2O(l) → H2SO4(l) + 130 kJ mole-1
In the commercial production of sulfuric acid, the contact process or the lead-chamber process is used. In the contact method, sulfur dioxide is catalytically converted to sulfur trioxide by surface chemistry with fine platinum powder or, more recently, vanadium pentoxide (V2O5). The resulting sulfur trioxide gas is bubbled through sulfuric acid and the addition of water at the correct rate yields 98% acid pulled out.
The lead-chamber process uses sulfur dioxide, oxygen, nitric acid and water vapor are introduced into a lead-lined chamber. White crystals of nitrosulfuric acid (nitronium sulfate), NOHSO4, are formed. The introduction of steam then converts the nitrosulfuric acid to sulfuric acid liberates nitrogen oxides, which can be reused in the first step of the reaction.
- 1) 2SO2 + NO + NO2 + O2 + H2O → 2NOHSO4
- 2) 2NOHSO4 + H2O → 2H2SO4 + NO + NO2
Soluble and insoluble sulfate salts
The soluble salts of sulfate include sodium sulfate (Na2SO4)•10H2O, ammonium sulfate (NH4)2SO4, magnesium sulfate (Epsom salt, MgSO4•7H2O, copper sulfate (blue vitriol, CuSO4•5H2O), iron sulfate (FeSO4•7H2O), zinc sulfate (ZnSO4•7H2O), potassium aluminium sulfate (alum, KAl(SO4)2•12H2O), ammonium aluminium sulfate (ammonium alum, NH4Al(SO4)2•12H2O), and chrome alum (KCr(SO4)2•12H2O).
Barium sulfate (barite) is the least soluble sulfate salt and its white precipitate is used as a test for sulfate anions. Other sulfates with diminished solubility include lead sulfate (PbSO4), strontium sulfate (SrSO4) and calcium sulfate (gypsum, CaSO4•2H2O.